Ion activity, mean activity coefficient
advertisement

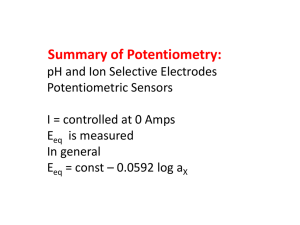
Ion activity, mean activity coefficient Activity (ai) of a molecule or ion in a solution is referred to as effective concentration and is related to concentration (Ci) by the simple relationship: ai i Ci Where i is known as an activity coefficient which may take different forms depending on the way in which concentrations for a given system are expressed, i.e. as morality, molality or mole fraction. For instance, the chemical potential ( i ) of a species i may be expressed i i RT ln i Ci For an ideal solutions or infinite diluted solutions ( i 1 ai Ci ): i i RT ln Ci Ion activity Activities of ionic solutions serve us for calculating accurate chemical potentials accurate equilibrium constants Ionic activities are more important than activities for non-electrolytes. Intermolecular forces are the greatest between ions. Ions in aqueous solution When ionic compounds (1:1) dissolve they are completely dissociated into ions. For example NaCl: NaCl( aq) Na(aq) Cl(aq) The chemical potentials for the formation of ions, μNaCl, aq μNa ,aq μCl ,aq Since o NaCl ( aq) NaCl ( aq) RT ln a NaCl ( aq) Na ( aq ) o Na RT ln a Na ( aq) ( aq ) Cl ( aq) Clo ( aq ) RT ln aCl ( aq) So, o o NaCl ( aq ) RT ln a NaCl ( aq ) Na ( aq ) Clo ( aq) RT ln a Na ( aq) RT ln aCl ( aq) Rearrangement ln a NaCl ( aq) ln a Na ( aq) ln aCl ( aq) ln a NaCl ( aq) ln Na C Na ln Cl CCl ln Na Cl C Na CCl a NaCl ( aq) Na Cl C Na CCl Since, Na Cl 2 C NaCl ( aq) C Na CCl 2 aNaCl ( aq) 2CNaCl The above equation is applicable for 1:1 electrolyte. Try MgCl2, i.e. 1:2 electrolyte Innovations MgCl2 → Mg2+ + 2 Cl− 0 0 salt RT ln asalt Mg RT ln C Mg 2 Cl0 2 RT ln CCl 2 2 0 0 salt RT ln asalt Mg 2 Cl0 RT ln C C 2 2 2 so asalt C C2 2 But for MgCl2 at molality, m, we know that C+ = C and C− = 2C. Further, we define the geometric mean activity coefficient by, 2 3 Then asalt C 2C 3 4C 3 3 2 Other ionic compounds are done in a similar manner. After some practice you can probably figure out the expression for asalt just by looking at the compound. Until then, or when in doubt, go back to the expressions for chemical potentials as we have done here. (For practice you might want to try Al2(SO4)3.). General formula Absolute activities of cations and anions can not be determined experimentally. The definition of the mean activity coefficient depends on the number of ions into which a molecule dissociates when it is dissolved. In the laboratory it is impossible to study solutions which only contain one kind of ion. Instead, solutions will have at least one positive and one negative type of ion. For the generic electrolytic compound dissolving in water: A x By xA z yBz a salt aAx aBy x x y y C x y x y For MgCl2 a MgCl2 a Mg aCl2 - 11 2 2 C 3 3 4C 3 3 The General Nernst Equation The general Nernst equation correlates the Gibb's Free Energy ΔG and the emf of a chemical system known as the galvanic cell. For the reaction aA bB cC dD and ac ad Q Ca Db a A aB For an ideal solutions a Concentration , because the activity coefficients equal to unity c d C D Q Aa Bb It has been shown that ΔG = ΔG° + R T ln Q and o o Since G nFEcell and G nFEcell Therefore o nFEcell nFEcell RT ln Q where R, T, Q and F are the gas constant (8.314 J mol-1 K-1), temperature (in K), reaction quotient, and Faraday constant (96485 C) respectively. Thus, we have RT C D ln nF Aa B b c Ecell E o cell d This is known as the Nernst equation. The equation allows us to calculate the cell potential of any galvanic cell for any concentrations. Some examples are given in the next section to illustrate its application. It is interesting to note the relationship between equilibrium and the Gibb's free energy at this point. When a system is at equilibrium, Ecell 0 , and Q K e . Therefore, we have, RT o Ecell ln K e nF RT aCc aDd o Ecell ln nF a Aa aBb For an ideal solution RT C D ln nF Aa B b c E o cell d o Thus, the equilibrium constant and Ecell are related. The Nernst Equation at 298 K At any specific temperature, the Nernst equation derived above can be reduced into a simple form. For example, at the standard condition of 298 K (25°), the Nernst equation becomes C D 0.059 log n Aa Bb c o Ecell Ecell d For the cell Zn | Zn2+ || H+ | H2 | Pt we have the following half cell reactions: o Zn( s ) Zn(2aq ) 2e (anode) eZn -0.763 2 / Zn 2 H (aq) 2e H 2 ( g ) (cathode) Zn( s ) 2 H ( aq ) 2 ( aq ) Zn H 2(g ) eHo / H 0 2 E o cell 0.763 If the concentrations of the ions are not 1.0 M, and the H2 pressure is not 1.0 atm, then the cell potential ΔE may be calculated using the Nernst equation: Ecell 0.059 Zn 2 P( H 2 ) 0.763 log 2 Zn H 2 with n = 2 in this case, because the reaction involves 2 electrons. The numerical value is 0.0592 only when T = 298 K. This constant is temperature dependent. Note that the reactivity of the solid Zn is taken as 1. If the H2 pressure is 1 atm, the term P(H2) may also be omitted. The expression for the argument of the log function follows the same rules as those for the expression of equilibrium constants and reaction quotients. Indeed, the argument for the log function is the expression for the equilibrium constant K, or reaction quotient Q. When a cell is at equilibrium, Ecell 0 and the expression becomes an equilibrium constant K, which bears the following relationship: o Ecell RT ln K e nF o where Ecell is the difference of standard potentials of the half cells involved. Problem 1 Calculate Ecell for the cell which is shown in the following diagram Pt H 2 (1.0 atm) HCl (0.5 M) AgCl Ag Where the temperature is 25 oC and mean activity coefficients of HCl is 0.758. Anodic reaction H 2 ( g ) 2H 2e Cathodic reaction 2 AgCl(s) 2e 2 Ag 2Cl 2 AgCl(s) H 2 2 Ag 2Cl 2 H Write Nernst equation Ecell E o cell 2 a Ag aCl2 aH2 0.059 log 2 2 a AgCl aH 2 0.059 log aCl2 a H2 2 2 0.059 o Ecell Ecell log aCl a H 2 o o o 2 2 Since aHCl aCl aH CHCl and Ecell ecathod eanode 0.223 o Ecell Ecell 2 Ecell 0.223 0.059 log 2C HCl Ecell 0.223 2 0.059 log( 0.758 0.5) Ecell 0.273 V Problem 2 The standard cell potential dE° for the reaction at 25 oC Fe + Zn2+ = Zn + Fe2+ is -0.353 V. If a piece of iron is placed in a 1 M Zn2+ solution, what is the equilibrium concentration of Fe2+? Solution The equilibrium constant K may be calculated using o Ecell RT ln K e nF K e 10 K e 10 o nEcell 0.059 o nEcell 0.059 K e 1.08 10 12 Fe2 2 1M , since K e 2 and Zn Zn Fe2 1.08 10 12 Problem From the standard cell potentials, calculate the solubility product for the following reaction: AgCl = Ag+ + ClSolution There are Ag+ and AgCl involved in the reaction, and from the table of standard reduction potentials, you will find: AgCl + e = Ag + Cl-, E° = 0.2223 V - - - -(1) Since this equation does not contain the species Ag+, you need, Ag+ + e = Ag, E° = 0.799 V - - - - - - (2) Subtracting (2) from (1) leads to, AgCl = Ag+ + Cl- . . . ΔE° = - 0.577 Let Ksp be the solubility product, and employ the Nernst equation, o Ecell RT ln K sp nF log Ksp = (-0.577) / (0.0592) = -9.75 Ksp = 10-9.75 = 1.8x10-10 This is the value that you have been using in past tutorials. Now, you know that Ksp is not always measured from its solubility. Variation of e.m.f with temperature One of the most rewarding aspects of electrochemistry is that ΔS values spring directly from studies of the temperature variation of e.m.f. Equation (V-28), to be derived in Chapter V, describes the temperature variation of G: G S T P For G o one can write G o S o T P Since G o nFE o So dG o dE o nF , Then dT dT dE o nF S o dT dE o S o dT nF Example Niobium oxides were studied by use of the cell: The net reaction: Nb NbO2 2NbO Values of e.m.f. at various temperatures are given in the following table: Temperature (K) Eo (mV) 1054 213 1086 212 1106 209 1160 201.5 1174 202 1214 200 1244 198 1283 194 5 -1 The temperature coefficient = 9 10 Volt K (A) Write the two half cell reactions? S o for the cell reaction. o o (C) Calculate the average value of G and H for the cell reaction? (B) Calculate Solution (A) Anodic reaction Nb O 2 NbO 2e Cathodic reaction NbO2 2e NbO O 2 The total reaction is Nb NbO2 2NbO (B) dE o nF S o dT S o 2 96485 (9 105 ) 17.37 J mol -1 K-1 (C) o o o o o o o G o nFECell and G H TS H G TS Temperature (K) Eo (mV) G o (kJ mol-1) H o (kJ mol-1) 1054 1086 1106 1160 1174 1214 1244 1283 213 212 209 201.5 202 200 198 194 41.10 40.91 40.33 38.88 38.98 38.59 38.21 37.44 39.31 59.41 59.77 59.54 59.54 59.37 59.68 59.82 59.73 59.61 Mean value