16_chp23_slides
advertisement

Measuring concentration using electrodes
Indicator electrodes used with reference electrode to measure potential
of unknown solution
Ecell = Eindicator – Ereference+ Ej (potential arising from salt bridge)
Eindicator
- responds to ion activity
- specific (one ion) or selective (several ions)
Two general types of indication electrodes
- metallic
- membrane
Fig. 23-1 (p.660) A cell for potentiometric determination
2.1 Electrodes of the first kind
- respond directly to activity of electrode ion
copper indicator electrode
Cu2+ + 2e- Cu(s)
Eind E
0
Cu 2
0.0592
1
log
2
aCu 2
0
ECu
2
Problems:
0.0592
pCu
2
simple but not very selective
some metal electrode can not be use in acidic solutions
some easily oxidized (deaerated solutions)
2.2 Electrodes of the second kind
- respond to anion activity through formation of complex
silver electrode works as halide or halide-like anions
AgCl(s) + e- Ag(s) + Cl- E0 = +0.222 V
0.0592
log aCl
2
0.222 0.0592pCl
Eind 0.222
mercury electrode works for EDTA (ethylene-diamine-tetra-acetic acid)
HgY2- + 2e- Hg (l) + Y4- E0 = +0.21 V Y4-: EDTA anion
Eind 0.21
a 4
0.0592
log Y
2
aHgY 2
0.0592
log aY 4
2
0.0592
K
pY
2
K
2.3 Electrodes of the third kind
- respond to different ion than metal electrode
mercury electrode works for EDTA
HgY2- + 2e- Hg (l) + Y4- E0 = +0.21 V
CaY2-Ca2+ + Y4- Kf = Ca2+Y4-/caY2-
Eind 0.21
a 4
0.0592
log Y
2
aHgY 2
0.0592
log aY 4
2
0.0592 K f aCaY 2
Klog
2
aCa 2
K
K-
0.0592
0.0592
1
log K f aCaY 2
log
2
2
aCa 2
K'-0.0592p
Ca
Membrane
Minimal solubility – solids, semi-solids and polymer
Some electrical conductivity
Selective reactivity with the analyte
Types (see Table 23-2 for examples)
Crystalline
Single crystal {LaF3 for F-}
Polycrystalline or mixed crystal: {Ag2S for S2- and Ag}
Noncrystalline
Glass:– {silicate glasses for H+, Na+}
- Liquid: {liquid ion exchange for Ca2+ }
3.1 Glass pH electrode
Contains two reference electrodes
Fig. 23-4 (p.666) Glass-calomel cell for pH measurement
Eind = Eb+Eref2
Ecell = Eind - Eref1
Combination pH electrode (ref + ind)
Fig. 23-3 (p.666) Glass pH electrode
Membrane structure
SiO4- frame work with charge balancing cations
In aqueous, ion exchange reaction at surface
H+ + Na+Glass- H+Glass- + Na+
H+ carries current near the surface
Na+ carries charge in interior
Fig. 23-4 (p.666) Silicate glass structure for a glass pH electrode
Boundary Potential Eb
Eb E1 E2
0.0592
a1'
E1 j1
log
n
a1
0.0592
a2'
E 2 j2
log
n
a2
j1 j2 , a1' a'2 ,a2 const ant
a1
Eb 0.0592log
a2
L'0.0592log a1 L' 0.0592pH
Difference compared with metallic electrode: the boundary potential depends
only on the proton activity
Asymmetry potential
Fig. 23-6 (p.669) Potential profile across a glass membrane
Boundary Potential Eb
Eb E1 E2
0.0592
a1'
E1 j1
log
n
a1
0.0592
a2'
E2 j2
log
n
a2
j1 j2 , a1' a'2 ,a2 constant
Eb 0.0592log
a1
L'0.0592log a1 L' 0.0592pH
a2
Eind L' 0.0592pH Eref 2 ( Ag / AgCl) Easy
Easy : calibration agianst standardsolutions
Ecell Eind E ref 1( SCE) constant 0.0592pH
Sources of uncertainty in pH measurement with glass-electrode
1. Alkaline error
Eind constant 0.0592log(aH k Na / H aNa )
k Na / H selectivity coefficient
2. Others {Problems, #23-8)
Glass electrodes for other ions (Na+, K+, Cs+,…):
- Minimize aH+
Maximize kH/NaNa+ for other ions
modifying the glass surface (incorporation of Al2O3 or B2O3)
Fig. 23-7 (p.670) Acid and alkaline error of selected glass electrode
3.2 Crystalline membrane electrode (optional)
-
-
Usually ionic compound
Single crystal
Crushed powder, melted and formed
Sometimes doped with Li+ to increase conductivity
Operation similar to glass membrane
Fluoride electrode
At the two interfaces, ionization creates a charge on the membrane surface as
shown by
LaF3 LaF2 F
Eind L 0.0592log aF L 0.0592pF
The magnitude of charge depend on fluoride ion concentration of the solution.
4.1 Gas sensing probes
simple electrochemical cell with
two reference electrodes and gaspermeable PTFE membrane
- allows small gas molecules to
pass and dissolve into internal
solution
- analyte not in direct contact with
electrode – dissolved
Fig. 23-12 (p.677) Schematic
of a gas-sensing probe for CO2
CO2 (aq) CO2 ( g ) CO2 (aq)
analyte
membrane
probe
int ernal
solution
in internal solution
assuming aHCO - constant
3
aH
K eq
aHCO
[CO2 ]
3
CO 2 (aq) H 2 O H HCO3-
can use glass membraneelectrodeto sense pH!
Overallequation
Eind L 0.0592log aH
CO 2 (aq) H 2O H HCO3
external analyte
K eq
int eranl solution
aH aHCO
3
aCO2
aCO2 [CO2 ] for gas activity
L 0.0592log
K eq
aHCO
[CO2 ]
3
L' 0.0592log[CO2 ]
E c ell Eind Eref
L' 0.0592log[CO2 ] Eref
L" 0.0592log[CO2 ]
rel error
EM Ecell
IRcell
Ecell
I ( RM Rcell )
Need high impedancedevice for measuringE cell
RM
EM Ecell
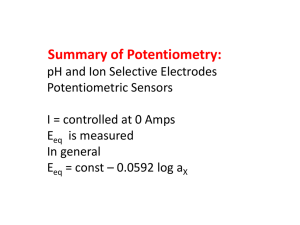
Sum m ary
Ecell Eind Eref
for cations
0.0592
pX
n
n( Ecell K )
pX
0.0592
E cell K
for anions
0.0592
E cell K
pA
n
n( Ecell K )
pA
0.0592