11.4: Empirical and Molecular Formulas
advertisement

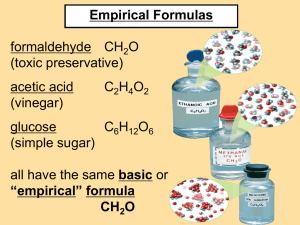
Table of Contents Chapter 11: More on the Mole 11.4: Empirical and Molecular Formulas 11.5: Hydrates 11.4: Empirical and Molecular Formulas • Percent composition: the percent by mass of each element in a compound % by mass= (moles element) x (mass of element) Mass of compound • The sum of the mass percents = 100% x100 11.4: Percent composition • What is the percent composition of H2O? H: (2mol H) x (1.01g/mol H) x 100 = 11.2% H 18.02 g H2O O: (1mol O) x (16.00g/mol O) x 100 = 88.80% O 18.02 g H2O • The percent composition of H2O is 11.2% H and 88.80% O. 11.4: Percent composition • What is the percent composition of NaHCO3? Na: (1mol Na) x (22.99g/mol Na) 84.01 g NaHCO3 H: (1mol H) x (1.008g/mol H) 84.01 g NaHCO3 C: (1mol C) x (12.01g/mol C) 84.01 g NaHCO3 O: (3mol O) x (16.00g/mol O) 84.01 g NaHCO3 x 100 = 27.37% Na x 100 = 1.2% H x 100 = 14.3% C x 100 = 57.14% O 11.4: Empirical and Molecular Formulas • Why? This process can be used to determine the composition of an unknown compound • Empirical Formula: the formula with the simplest whole number ratios 11.4: Empirical and Molecular Formulas Finding Empirical Formulas • You have an substance made of 40.05 % Sulfur and 59.95% Oxygen. What is its empirical formula? 1) Assume percentages are masses • S = 40.05 g • O = 59.95 g 11.4: Empirical and Molecular Formulas You have an substance made of 40.05 % Sulfur and 59.95% Oxygen. What is its empirical formula? 2) Convert grams to moles → ratio • S = 40.05 g 1 mol S 32.07 g S • O = 59.95 g 1 mol O 16.00 g O = 1.249 mol S = 3.747 mol O • Ratio S : O = 1.249 : 3.747 11.4: Empirical and Molecular Formulas You have an substance made of 40.05 % Sulfur and 59.95% Oxygen. What is its empirical formula? 3) Simplify ratio: divide each by smallest moles • S = 1.249 mol S 1.25 = 1 mol S • O = 3.747 mol O = 3 mol O 1.25 • Empirical formula: SO3 11.4: Empirical and Molecular Formulas EXAMPLE 1: Determine the empirical formula for a compound with 48.64 % C, 8.16% H, and 43.20% O. 1) Assume percentages are masses • C = 48.64 g • H = 8.16 g • O = 43.20 g 11.4: Empirical and Molecular Formulas EXAMPLE 1: Determine the empirical formula for a compound with 48.64 % C, 8.16% H, and 43.20% O. 2) Convert grams to moles → ratio • C = 48.64 g 1 mol C 12.01 g C • H = 8.16 g 1 mol H 1.008 g H • O = 43.20 g 1 mol O 16.00 g O = 4.050 mol C = 8.10 mol H = 2.700 mol O 11.4: Empirical and Molecular Formulas EXAMPLE 1: Determine the empirical formula for a compound with 48.64 % C, 8.16% H, and 43.20% O. 3) Simplify ratio: divide each by smallest moles • C = 4.050 mol C = 1.5 mol C 2.700 • H = 8.10 mol H 2.700 = 3 mol H • O = 2.700 mol O = 1 mol O 2.700 11.4: Empirical and Molecular Formulas EXAMPLE 1: Determine the empirical formula for a compound with 48.64 % C, 8.16% H, and 43.20% O. 3) Simplify ratio: whole numbers • C = 1.5 mol x 2 = 3 mol C • H = 3 mol x 2 = 6 mol H • O = 1 mol x 2 = 2 mol O • Empirical formula: C3H6O2 11.4: Empirical and Molecular Formulas Practice: Ex 2) A blue solid has 36.84% nitrogen and 63.16% Oxygen. What is its empirical formula? N2O3 Ex 3) Aspirin contains 60.00% carbon, 4.44% hydrogen, and 35.56% oxygen. What is its empirical formula? C9H8O4 11.4: Empirical and Molecular Formulas • Sometimes substances with different properties have the same percent composition and empirical formula. WHAT? • Molecular Formula: the actual number of atoms in a molecule/formula unit of a substance 11.4: Empirical and Molecular Formulas Finding Molecular Formulas • The molar mass of acetylene is 26.04 g/mol. The mass of its empirical formula (CH) is 13.02 g/mol 1) Divide: correct molar mass mass of empirical formula = 26.04 g/mol 13.02 g/mol = 2 So… the molecular formula is 2 times the empirical formula 11.4: Empirical and Molecular Formulas Finding Molecular Formulas • The molar mass of acetylene is 26.04 g/mol. The mass of its empirical formula (CH) is 13.02 g/mol 2) Multiply: CH x 2 = C2H2 11.4: Empirical and Molecular Formulas Finding Molecular Formulas EXAMPLE 4: Succinic acid has a molar mass of 118.1 g/mol and an empirical formula of C2H3O2 1) Divide: correct molar mass mass of empirical formula = 118.1 g/mol 59.04 g/mol = 2 So… the molecular formula is 2 times the empirical formula 11.4: Empirical and Molecular Formulas Finding Molecular Formulas EXAMPLE 4: Succinic acid has a molar mass of 118.1 g/mol and an empirical formula of C2H3O2 2) Multiply: C2H3O2 x 2 = C4H6O4 11.4: Empirical and Molecular Formulas Practice: Ex 5) A photographic developing fluid has a chemical composition of 65.45% C, 5.45% H, and 29.09% O. Its molar mass is 110.0 g/mol. What is its molecular formula? (Find its empirical formula first) C6H6O6 Ex 6) A colorless liquid is composed of 46.68% nitrogen and 53.32% oxygen has a molar mass of 60.01 g/mol. What is the molecular formula? N2O2