Chapter 15 – Acid-Base Equilibria
advertisement
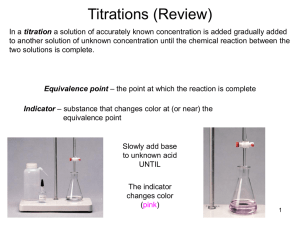
Acid-Base Equilibria • • • • • Common Ion Effect in Acids and Bases Buffer Solutions for Controlling pH Buffer Capacity pH-Titration Curves Acid-Base Titration Indicators Common Ion Effect • Shift in the equilibrium position due to the addition of an ion already involved in the equilibrium process. • An application of Le Châtelier’s principle. A Common Ion Effect Consider the following equilibrium: • HC2H3O2(aq) + H2O(l) ⇌ H3O+(aq) + C2H3O2-(aq) • • • Adding NaC2H3O2 to the solution will shift the equilibrium to the left because [C2H3O2-] increases; C2H3O2- is part of the equilibrium system. This equilibrium shift causes [H3O+] to decrease and raise the pH of the solution. Solutions containing a mixture of HC2H3O2 and NaC2H3O2 are less acidic than those solutions of HC2H3O2 alone, and they are less basic than those of NaC2H3O2 alone. pH of weak acid and the Effect of Common Consider the following solutions: • Calculate the pH of 1.00 M HC2H3O2 solution. • What is the pH of a solution that contains 1.00 M HC2H3O2 and 0.50 M NaC2H3O2. Solution-1: Equilibrium: HC2H3O2(aq) ⇌ H+(aq) + C2H3O2-(aq) Initial [ ], M 1.00 0.00 0.00 Change, D[ ], M -x +x +x Equilm. [ ], M (1.00 – x) x x pH of Acetic Acid by itself. • Solution-1: Ka [H 3 O ][CH 3 CO 2 ] [CH 3 COOH] • By approximation, x = x 2 1.8 x 10 (1.00 - x ) (1 . 00 x 1.8 x 10 ) 4.2 x 10 -5 • [H3O+] = x = 4.2 x 10-3 M, pH = 2.37 -3 -5 Acetic Acid-Acetate Equilibrium Solution-2: Equilibrium: HC2H3O2(aq) ⇌ H+(aq) + C2H3O2-(aq) Initial [ ], M 1.00 0.00 0.50 Change, D[ ], M -x +x +x Equilm. [ ], M (1.00 – x) x (0.50 + x) pH of Acetic Acid + Sodium Acetate Solution-2: Ka [H 3 O ][CH 3 CO 2 ] [CH 3 COOH] ( x )( 0 . 50 x ) 1.8 x 10 -5 (1.00 - x ) By approximation, x = (1.00/0.50)(1.8 x 10-5) = 3.6 x 10-6 M [H+] = x = 3.6 x 10-6 M, pH = 4.44 Solution containing HC2H3O2 and NaC2H3O2 is less acidic than one containing only HC2H3O2 at the same concentration. Solving Problems with Buffered Solutions Buffering: How Does It Work? Buffering: How Does It Work? Buffer Solutions Henderson–Hasselbalch Equation • HA(aq) ⇌ H+(aq) + A-(aq); • Ka = [ H ][ A ] [ HA ] • pH = pKa + log([A–]/[HA]) • For a particular buffer system, solutions with the same [A–]/[HA] ratio have same pH. pH of Buffer Solution: example #1 • What is the pH of a buffer solution that is 0.45 M acetic acid (HC2H3O2) and 0.85 M sodium acetate (NaC2H3O2)? The Ka for acetic acid is 1.8 × 10–5. • • • • Solution: pH = pKa + log([C2H3O2-]/[HC2H3O2] pH = -log(1.8 × 10–5) + log(0.85/0.45) pH = 4.74 + 0.28 = 5.02 Characteristics of Buffer Solutions • Contain weak acids or weak bases and their corresponding conjugate partners (common ions). • Resist changes in pH. • Buffering capacity depends on concentrations of weak acid or weak base and their common ions. • Effective pH buffering range ~ pKa 1 Characteristics of Buffer Solutions 1. 2. 3. 4. Buffers contain relatively large amounts of the weak acids (HA) and their conjugate base (A)־, (or weak bases and their conjugate acids) Buffer pH is determined by the pKa of the acid HA and the molar ratio of the conjugate base to acid: [A]־/[HA]. Buffer pH changes very little because the ratio [A]־/[HA] changes very little when a small amount of strong acid or strong base is added. [H3O+] in buffer solutions remains more or less constant: Most of H+ from strong acid is absorbed by the conjugate base A ;־most of OH ־added from strong base reacts with acid HA in the buffer to yield A ־and H2O. Buffering Capacity • How much H3O+ or OH- the buffer can absorb without significantly changing its pH. • Depends on the concentrations of HA and A־. • High [HA] and [A ]־lead to large buffering capacity. • Optimal buffering occurs when [HA] = [A;]־ • Ratio [A–] / [HA] ~ 1 strong resist to change when either H3O+ or OH– is added. Some Common Buffers Buffers pKa pH Range • HCHO2 – NaCHO2 3.74 2.74 – 4.74 • CH3CO2H – NaCH3CO2 4.74 3.74 – 5.74 • KH2PO4 – K2HPO4 7.21 6.20 – 8.20 • CO2/H2O – NaHCO3 6.37 5.40 – 7.40 • NH4Cl – NH3 9.25 8.25 – 10.25 Choosing a Buffer System • The weak acid in buffer has pKa close to target pH. • For example, KH2PO4 and K2HPO4 may be used to buffer at pH ~ 7.5 (H2PO4 ־has pKa = 7.20) • Phosphate buffer is most effective in the pH range 6.20 – 8.20; it has the highest buffering capacity at about pH = 7.20. Making Buffer Solution: example #2 A phosphate buffer with pH = 7.40 is prepared using KH2PO4 and K2HPO4. (a) What is the molar ratio of [HPO42-] to [H2PO4-] in the buffered solution? (b) If [H2PO4-] = 0.20 M, what is [HPO42-]? (c) How many grams of KH2PO4 and K2HPO4, respectively, are needed to make 500. mL of this solution? (H2PO4- has Ka = 6.2 x 10-8) Solutions to Buffer example #2 (a) Use Henderson-Hasselbalch equation: • pH = pKa + log([HPO42-]/[H2PO4-]) • 7.40 = 7.21 + log([HPO42-]/[H2PO4-]) • log([HPO42-]/[H2PO4-]) = 7.40 – 7.21 = 0.19 • [HPO42-]/[H2PO4-] = 100.19 = 1.55 (b) If [H2PO4-] = 0.20 M, • [HPO42-] = 1.55 x 0.20 M = 0.31 M Solutions to Buffer example #2 (c) Moles of KH2PO4 needed = 500. mL x (1 L/1000 mL) x 0.20 mol/L = 0.10 mole • Moles of K2HPO4 needed = 500. mL x (1 L/1000 mL) x 0.31 mol/L = 0.155 mole • Grams of KH2PO4 needed = 0.10 mol x (136.086 g/mol) = 14 g • Grams of K2HPO4 needed = 0.155 mol x (174.178 g/mol) = 27 g Buffer Exercise #1 Indicate whether each of the following mixtures makes a buffer solution. Explain. (a) 50.0 mL of 0.20 M CH3CO2H + 50.0 mL of 0.20 M NaCH3CO2; (b) 50.0 mL of 0.20 M HC2H3O2 + 50.0 mL of 0.10 M NaOH; (c) 50.0 mL of 0.20 M HC2H3O2 + 50.0 mL of 0.20 M NaOH; (d) 50.0 mL of 0.20 M NaC2H3O2 + 50.0 mL of 0.20 M HCl; (e) 50.0 mL of 0.20 M NaC2H3O2 + 50.0 mL of 0.10 M HCl (Answer: (a) Yes; (b) Yes; (c) No; (d) No; (e) Yes) Buffer Exercise #2 Indicate whether each of the following solution mixtures will make a buffer solution. Explain. (a) 50.0 mL of 0.10 M NH3 + 50.0 mL of 0.10 M NH4NO3; (b) 50.0 mL of 0.10 M NH3 + 50.0 mL of 0.10 M HNO3; (c) 50.0 mL of 0.10 M NH3 + 25.0 mL of 0.10 M HNO3; (d) 50.0 mL of 0.10 M NH4NO3 + 25.0 mL of 0.10 M NaOH; (e) 50.0 mL of 0.10 M NH4NO3 + 50.0 mL of 0.10 M NaOH; (Answer: (a) Yes; (b) No; (c) Yes; (d) Yes; (e) No) Buffer Exercise #3 An acetate buffer solution is prepared by mixing 35.0 mL of 1.0 M acetic acid and 65.0 mL of 1.0 M sodium acetate. (a) What is the pH of this solution? (b) If 0.010 mole of HCl is added to this solution without altering its volume, what will be the pH of the resulting solution? (Ka = 1.8 x 10-5) (Answer: (a) pH = 5.01; (b) pH = 4.83 after adding 0.10 M HCl) Buffer Exercise #4 The Ka values of some acids and base are given below: 1. Acetic acid, CH3CO2H, Ka = 1.8 x 10-5; 2. Dihydrogen phosphate, H2PO4־, Ka = 6.2 x 10-8; 3. Ammonia, NH3, Kb = 1.8 x 10-5; 4. Hydrogen carbonate, HCO3־, Kb = 2.3 x 10-8. What solutions are used to make buffers with the following pH’s? (i) pH = 7.00; (ii) pH = 4.50; (iii) pH = 9.00 (iv) pH = 9.50; (v) pH = 5.00 Buffer Exercise #5 How many milliliters of each solution of 0.50 M KH2PO4 and 0.50 M K2HPO4 are needed to make 100.0 mL solution of phosphate buffer with pH = 7.50? What are the final concentrations of K+, H2PO4and HPO42-, in the buffer solution? (for H2PO4-, Ka = 6.2 x 10-8) (Answer: (a) 33.9 mL of KH2PO4 + 66.1 mL of K2HPO4; (b) [K+] = 0.83 M; [H2PO4-] = 0.17 M; [HPO42-] = 0.33 M) Titration and pH Curves • Plotting the pH of the solution being analyzed as a function of the amount of titrant added. • From pH-titration curve determine the equivalence point – when enough titrant has been added to react exactly with the substance in solution being titrated. The pH Curve for the Titration of 50.0 mL of 0.200 M HNO3 with 0.100 M NaOH The pH Curve for the Titration of 100.0 mL of 0.50 M NaOH with 1.0 M HCI The pH Curve for the Titration of 50.0 mL of 0.100 M HC2H3O2 with 0.100 M NaOH The pH Curves for the Titrations of 50.0-mL Samples of 0.10 M Acids with Various Ka Values with 0.10 M NaOH The pH Curve for the Titration of 100.0mL of 0.050 M NH3 with 0.10 M HCl Acid-Base Indicators • An indicator is a substance added to acid or base solution to marks the end point of a titration by the change of its color. For example, phenolphthalein changes from colorless to pink at the end point when an acid is titrated with a base. • The end point of a titration should correspond to the equivalence points of the acid-base reaction. The Acid and Base Forms of the Indicator Phenolphthalein The Methyl Orange Indicator is Yellow in Basic Solution and Red in Acidic Solution Choosing Indicators 1. The pH range for color changes should occur within the sharp vertical rise (or drop) in the pH-titration curves. 2. An indicator changes color at pH = pKa ± 1, where pKa is that of the indicator used. pH Ranges for Indicators Common Indicators Indicators: Acid Base pH Range Type of Titration Color Color 1. Methyl orange Orange Yellow 3.2 – 4.5 strong acid-strong base strong acid-weak base 2. Bromocresol Yellow Blue 3.8 – 5.4 strong acid-strong base green strong acid-weak base 3. Methyl red Red Yellow 4.5 – 6.0 strong acid-strong base strong acid-weak base 4. Bromothymol Yellow Blue 6.0 – 7.6 strong acid-strong base blue 5. Phenol Red Orange Red 6.8 – 8.2 strong acid-strong base weak acid-strong base Useful pH Ranges for Several Common Indicators Calculating the pH of solution during titration Strong Acid-Strong Base Titration; 1. Net reaction: H3O+(aq) + OH-(aq) 2H2O 2. Determine the limiting reactant and calculate the final concentration of H3O+ or OH- that is in excess. 3. Calculate pH using concentration of excess H3O+ or OH- Titration Problem: example #1 A 20.0 mL aliquot of 0.100 M HCl is titrated with 0.100 M NaOH solution. What is the pH of the resulting solution after 15.0 mL of NaOH has been added? Reaction: H3O+(aq) [I]before rxn: 0.057 M [C]from rxn: -0.043 M [E]after rxn: 0.014 M + OH-(aq) 2H2O 0.043 M -0.043 M 0.000 pH = -log(0.014) = 1.85 pH of Weak Acid-Strong Base Titrations Net reaction: HA(aq) + OH-(aq) H2O + A-(aq); 1. Assume the reaction with OH- goes to completion; 2. If OH- is the limiting reactant: (mol of HA)final = (mol of HA)initial – (mol of OH-); (mol of A-)final = (mol of OH-) 3. [HA]final = (mol of HA)final/Vfinal; 4. [A-]final = (mol A-)final/Vfinal 5. pH = pKa + log([A-]f/[HA]f) Titration Problem: example #2 Weak Acid-Strong Base Titration: • A 20.0 mL aliquot of 0.100 M HNO2 is titrated with 0.100 M NaOH. (a) What is the pH of the solution before titration? (b) What is the pH of the solution after 15.0 mL of NaOH has been added? (c) What is the pH of the solution at equivalent point (after 20.0 mL of 0.100 M NaOH is added)? (Ka of HNO2 = 4.0 x 10-4) Solution to Titration Problem: example #2 (a) Solving initial concentration of H3O+ by approximation method: [ H 3O ] [HNO ]x Ka 2 (0.100 x 4.0 x 10 pH = -log(0.0063) = 2.20 -4 0.0063 M Solution to Titration Problem: example #2 (b) Concentrations after 15.0 mL of NaOH is added: Reaction: HNO2(aq) + OH-(aq) NO2-(aq) + H2O [I]before rxn: 0.057 M 0.043 M 0.000 [C]from rxn: -0.043 M -0.043 M +0.043 M [E]after rxn: 0.014 M 0.000 0.043 M • pH = pKa + log([NO2-]f/[HNO2]f) • = -log(4.0 x 10-4) + log(0.043/0.014) • = 3.40 + 0.49 = 3.89 Solution to Titration Problem: example #2 (c) Calculating pH at equivalent point: Reaction: HNO2(aq) + OH-(aq) NO2-(aq) + H2O [I]before rxn: 0.050 M 0.050 M 0.000 [C]from rxn: -0.050 M -0.050 M +0.050 M [E]after rxn: 0.000 M 0.000 0.050 M • At equivalent point, [NO2-] = 0.050 M • Kb for NO2- = Kw/Ka = (1.0 x 10-14)/(4.0 x 10-4) = 2.5 x 10-11 Solution to Titration Problem: example #2 (c) Calculating pH at equivalent point (continue): • Set up the following equilibrium for the reaction of NO2- with water: • Reaction: NO2-(aq) + H2O ⇄ HNO2 + OH-(aq); [I]before rxn: 0.050 M [C]from rxn: -x [E]after rxn: (0.050 – x) 0.000 +x x 0.000 +x x Solution to Titration Problem: example #2 (c) Calculating pH at equivalent point (continue): Kb = x2/(0.050 – x) = 2.5 x 10-11 • x = [OH-], • Using approximation method, [ OH ] [NO 2 ] x K b - (0.050 x 2.5 x 10 • pOH = -log(1.1 x 10-11) = 5.95 • pH = 14.00 – 5.95 = 8.05 -11 1.1 x 10 -6 M pH of Strong Acid-Weak Base Titrations Net reaction: B(aq) + H3O+(aq) BH+(aq) + H2O; 1. Assume the reaction with H3O+ goes to completion; 2. If H3O+ is the limiting reactant, at the end of the reaction, (mol B)final = (mol B)initial – (mol H3O+); 3. (mol BH+)final = (mol H3O+) 4. [B]final = (mol B)final/Vfinal; 5. [BH+]final = (mol BH+)final/Vfinal 6. pH = pKa + log([B]f/[BH+]f; (pKa is for BH+) Titration Problem: example #3 Strong Acid-Weak Base Titration: • A 20.0 mL aliquot of 0.100 M NH3 is titrated with 0.100 M HCl. (a) What is the pH of the solution before titration? (b) What is the pH of the solution after 10.0 mL of HCl has been added? (c) What is the pH of the solution at equivalent point (after 20.0 mL of 0.100 M HCl is added)? (Kb of NH3 = 1.8 x 10-5) Solution to Titration Problem: example #3 (a) Solving initial concentration of OH- by approximation method: [OH ] - [ NH 3 ] x K b (0.100 x 1.8 x 10 ) 1.3 x 10 -5 -3 M [H3O+] = Kw/[OH-] = (1.0 x 10-14)/(1.3 x 10-3) = 7.5 x 10-12 M pH = -log(7.5 x 10-12 M) = 11.13 Solution to Titration Problem: example #3 (b) Concentration after 10.0 mL of HCl is added: Reaction: NH3(aq) + H3O+(aq) NH4+(aq) + H2O [I]before rxn: 0.067 M 0.033 M 0.000 [C]from rxn: -0.033 M -0.033 M +0.033 M [E]after rxn: 0.034 M 0.000 0.033 M • pH = pKa + log([NH3]f/[NH4+]f) • = -log(5.6 x 10-10) + log(0.034/0.033) • = 9.25 + (0.0) = 9.25 Solution to Titration Problem: example #3 (c) Calculating pH at equivalent point: Reaction: NH3(aq) + H3O+(aq) NH4+(aq) + H2O [I]before rxn: 0.050 M 0.050 M 0.000 [C]from rxn: -0.050 M -0.050 M +0.050 M [E]after rxn: 0.000 M 0.000 0.050 M • At equivalent point, [NH4+] = 0.050 M • Ka for NH4+ = Kw/Kb = (1.0 x 10-14)/(1.8 x 10-5) = 5.6 x 10-10 Solution to Titration Problem: example #3 (c) Calculating pH at equivalent point (continue): • Set up the following equilibrium for the reaction of NO2- with water: • Reaction: NH3(aq) + H3O+(aq) NH4+(aq) + H2O [I]before rxn: 0.050 M [C]from rxn: -x [E]after rxn: (0.050 – x) 0.000 +x x 0.000 +x x Solution to Titration Problem: example #3 (c) Calculating pH at equivalent point (continue): Ka = x2/(0.050 – x) = 5.6 x 10-10 • x = [H3O+], • Using approximation method, [H 3 O ] [NH 4 ]x Ka (0.050 x (5.6 x 10 • pH = -log(5.3 x 10-6 = 5.28 -10 ) 5.3 x 10 -6 M Titration Exercise #1 25.0 mL of 0.10 M HCl is titrated with 0.10 M NaOH solution. (a) What is the pH of the acid before NaOH solution is added? (b) What is the pH after 15.0 mL of NaOH solution is added? (c) What is the pH of the solution after 25.0 mL of NaOH is added? (Answer: (a) pH = 1.00; (b) pH = 1.60; (c) pH = 7.00) Titration Exercise #2 25.0 mL of 0.10 M acetic acid is titrated with 0.10 M NaOH solution. (a) What is the pH of the acid solution before NaOH is added? (b) What is the pH after 15.0 mL of NaOH solution is added? (c) What is the pH after 25.0 mL of NaOH is added? (Answer: (a) pH = 2.87; (b) pH = 4.92; pH = 8.72) Titration Exercise #3 25.0 mL of 0.10 M lactic acid, HC3H5O3, is titrated with 0.10 M NaOH solution. After 15.0 mL of NaOH is added, the solution has pH = 4.03. (a) Calculate the Ka of lactic acid. (b) What is the initial pH of 0.10 M lactic acid before NaOH is added? (Answer: (a) Ka = 1.4 x 10-4; (b) pH = 2.43)