Exp 5
advertisement

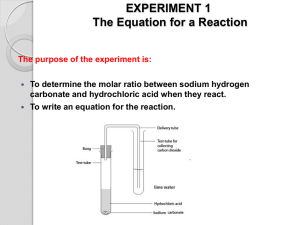
Plan for Tues/Wed, 28/29 Oct 08 • Turn in Exp 4 Formal Report and Exp 5 Pre-lab • Today: Exp 5, Gas Laws • Purpose: – To employ the Ideal Gas Law and Dalton’s Law of partial pressures to determine the number of moles of CO2(g) produced in the reaction: HCl(aq) + NaHCO3(s) H2O(l) + CO2(g) + NaCl(aq) – To determine the mass-% of sodium bicarbonate in a sample of the antacid Alka Seltzer. – To use these techniques to determine the mass-% of calcium carbonate in an unknown sample. 2 HCl(aq) + CaCO3(s) H2O(l) + CO2(g) + CaCl2(aq) Tips for success TECHNIQUE • DO NOT let the level of water in your buret fall below the markings!! • MAKE SURE your experimental set-up is free of leaks!! • MAKE SURE the water levels in your buret and leveling bulb are equal before the reaction occurs. • DO NOT disconnect any tubing in your set up while there is still water in your buret. Pour the water out through the leveling bulb. PROCEDURE • Obtain two consistent trials, within 5-10% of each other, not within 5-10% of the expected mass-% of NaHCO3 in Alka Seltzer WASTE • Acetone rinse should be disposed of in the liquid waste container. • Excess HCl(aq) may be poured down the drain and followed with 10 -20 • NEVER put a spatula, etc into a reagent bottle. NEVER return unused reagent to the reagent bottle. Any unused reagent must be placed in the waste container. • DO NOT overfill the liquid waste containers. Let me know if they are getting too full and I will replace them. SAFETY • HCl(aq) can cause chemical burns!! BE CAREFUL!! Gloves are available at the back of the lab. Inform me or the lab staff of any acid spills • Be sure to wash and dry your lab bench to remove all traces of any spilled chemicals. • Wash your hands with soap and water before you leave lab. Vapor Pressure • Some molecules at the surface of a sample of liquid have enough kinetic energy to enter the vapor phase, even if the liquid is not boiling. • This is why a glass of water left open will eventually completely evaporate. • This is an example of vapor pressure, the magnitude of which depends on the temperature of the liquid. • As T goes up, Pvap also goes up. Collecting gases over water • Gaseous products of reactions are often measured by the displacement of water. • The pressure of the gas inside the collection tube is a sum of the pressures of the product gas and of the water: Ptotal = Pproduct + PH2O • If you know the total P (usually atmospheric P), and PH2O, you can find Pproduct. Determining the total pressure • Your experimental set-up is similar to a manometer. Pgas < Patm h Pgas > Patm h If the level of the mercury in each side of the tube were the same, the trapped gas would be at the same pressure as Patm. In your set-up, you are using DI water rather than Hg, and you can manually adjust the water level by moving the leveling bulb up or down. Determining Moles of CO2(g) • From your experimental set-up, you will obtain the volume of CO2(g) produced. • Using this value, the atmospheric pressure, the temperature of the water, and the vapor pressure of water at that temperature, you can calculate the number of moles of CO2(g). Temperature of the water PCO2 = Patm – PH2O PCO2 V = nCO2 RT Displacement of water in buret What you want Determining Mass-% NaHCO3 in Alka Seltzer • Using the moles of CO2 you just determined and the chemical equation below, you can find the mass of NaHCO3(s) in your original sample of Alka Seltzer. HCl(aq) + NaHCO3 (s) H2O(l) + CO2 (g) + NaCl(aq) y g NaHCO3 = x mol CO2 1 NaHCO3 84.01 g NaHCO3 1 CO2 1 mol NaHCO3 • Mass Percent: Mass-% NaHCO3 = y g NaHCO3 Mass of your Alka Seltzer sample X 100