Exp. 10: Antiacid Analysis
advertisement

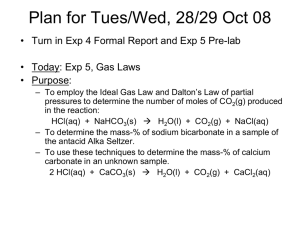
Exp. 10: Antiacid Analysis Required Locker Materials: - Gas apparatus (see figure) - Small vial - Standard test tube Safety: - Eyewear MUST be worn at all times Waste: - All liquid waste must be disposed in the waste containers. Experimental Objective: The object of the lab is to determine the percentage of active anti-acid compound in a commercially available antiacid medication. Experimentally this will be done by using a gas collection apparatus to collect the carbon dioxide produced in the reaction of the antiacids with hydrochloric acid. Students will again learn to combine a stoichiometry problem with a gas law problem. In addition, students will also deal with vapor pressures and partial pressure when doing the calculations. Introduction: Many commercial, over-the –counter antacids contain either a carbonate or a bicarbonate (i.e., hydrogen carbonate) compound as the active ingredient. For example, Alka Seltzer® contains NaHCO3 and Tums® contains CaCO3. When these antacids reach the stomach, the following reaction with gastric juice (which is mostly 0.2 M HCl) occurs: Alka Seltzer: NaHCO3 + HCl Tums: CaCO3 + 2HCl NaCl + H2O + CO2 CaCl2 + H2O + CO2 Procedure For this experiment record all observations and measurements in your notebook: 1. Obtain about 200 mL to tap water in a beaker and mix in about 5 mL of 3 M HCl. 2. Dissolve approximately 1.0 g of NaHCO3 (watch out for fizzing overflow). This will saturate the mixture with CO2. 3. Use the solution to fill a leveling bulb/buret assembly as shown in Figure 10-1, except raise the leveling bulb higher. Set the water level in the buret near the 5 mL mark. 4. Obtain 25 mL of 3 M HCl. Dissolve 0.2 g of NaHCO3 in order to saturate it with CO2. (Keep this solution stoppered when not in use). 5. Measure about 5 mL of this solution into a medium-sized test tube, and position it next to the gas buret assemble. Exp. 10: Page 1 of 3 Leo Truttmann – Fall 13 6. Without touching the tablet directly, break an antacid tablet (note the brand) into small pieces and accurately weigh (to the nearest ±1mg)a sample in the range 0.2 to 0.3 grams, placing the sample inside a small vial. 7. Carefully, nest the vial inside the test tube containing the CO2-saturated HCl solution. 8. Stopper the rest tube, re-equilibrate the water levels in the leveling bulb and buret near the 5ml mark, if necessary, and record the initial volume in the buret (±0.1 mL). 9. To initiate the reaction, tilt the test tubes so that the contents mix efficiently. 10. When the fizzing reaction has subsided, re-align the leveling bulb until the water levels are at same pressure. Tap the test tube to dislodge any bubbles, then equilibrate the water levels and record the final volume (± 0.1 mL). 11. Calculate the volume difference as VCO2 produced from the tablet. 12. Record the barometric pressure (Pair) and room temperature (assume it is the same as the temperature of the CO2 being collected). 13. Repeat the experiment twice more with accurately-weighed pieces of the tablet. (reset the water level near the 5 ml mark when restarting the experiment.) Calculations For the calculation start a new section in your notebook. Remember you need to show every calculation you do by writing down the equation and an example calculation. The example calculation only needs to be done for one trial. The results of the remaining trials can be written down in a table in your notebook. For the next Lab meeting organize all your data and calculations in an excel table which is due at the beginning of the Lab. Volume of carbon dioxide The volume of carbon dioxide is the difference between your initial and final volume reading with your gas buret. Vapor pressure of water The vapor pressure of water only depends on the temperature. Read the vapor pressure from a table based on the measured room temperature. Make sure you write down the correct unit. Partial pressure of carbon dioxide The pressure of carbon dioxide in your apparatus is less than the atmospheric pressure in the lab. The difference is that nitrogen was collected over water which exerts a small pressure called vapor pressure. Moles of carbon dioxide With the ideal gas equation the number of moles of carbon dioxide can be calculated. Mass of NaHCO3 or CaCO3 With the number of moles known, the mass can be calculated by using the molar mass of the corresponding compound. % of NaHCO3 or CaCO3 in original tablet Calculate the percentage of the active ingredient in the initially measured tablet. Exp. 10: Page 2 of 3 Leo Truttmann – Fall 13 Average % and Average Deviation of the % mass Calculate the average mass percentage of your trial and the average Deviation. Measurements Trial 1 Mass of tablet [g] Mass sodium nitrite [ml] Volume initial [ml] Volume final [ml] Temperature [C] Barometric pressure [mmHg] Exp. 10: Page 3 of 3 Trial 2 Leo Truttmann – Fall 13 Trial 3