Alka-Seltzer Stoichiometry Lab: NaHCO3 Percent by Mass
advertisement

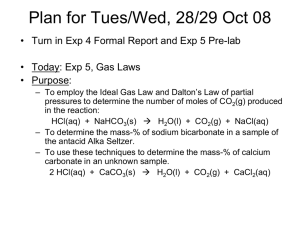
Laboratory in General Chemistry Fall 2009 Stoichiometry: Determination of Percent by Mass of NaHCO3 in Alka-Seltzer Tablets (modified from Chen & Yuang, 2002) I. Introduction Alka-Seltzer is an over-the-counter antacid and pain relief medication that is taken by dissolving it in water before ingesting. Alka-Seltzer is an effervescent tablet that contains aspirin (acetylsalicylic acid), citric acid, and NaHCO3. As soon as the tablet dissolves in water to form Na+ and the bicarbonate ion (HCO3-): NaHCO3 Na+ + HCO3- [Rxn 1], an acid-base reaction involving bicarbonate takes place: H+ + HCO3- H2CO3 H2O + CO2 [Rxn 2](the H+ comes from the “autoionization” of water: H2O H+ + OH-). The generation of carbon dioxide from the acid-base reaction causes the familiar bubbling of the Alka-Seltzer tablets. The release of carbon dioxide into the atmosphere results in a total weight loss after the reaction. With the weight loss, you should be able to calculate the amount of sodium bicarbonate reacted, and then determine the percent by mass of NaHCO3 contained in Alka Seltzer tablets. According to Rxns 1 and 2, it takes one mole of NaHCO3 to produce one mole of CO2. In other words, the moles of NaHCO3 reacted equal the moles of CO2 produced. Therefore, by calculating the number moles of CO2 produced we know the number of moles of NaHCO3 in a tablet. Then, by converting moles of NaHCO3 to mass of NaHCO3, in grams, we can calculate the percent by mass of NaHCO3 contained in Alka-Seltzer tablets. The second reaction shown above requires H+ for the reaction to proceed to the right. If H+ is limiting, not all of the HCO3- will be converted to CO2. We will use vinegar (5% acetic acid) to provide additional H+ and therefore convert all HCO3- to CO2. II. Materials and Equipment 8 (or more) Alka-Seltzer Tablets (Bayer Corporation) Vinegar (acetic acid, ≈ 5 %), 150 mL 250 mL beaker Electronic balance Graduated cylinder o To improve the results for the early trials use a 10 mL graduated cylinder 1 III. Experimental Procedure 1. add 35 mL of deionized water to a beaker o record the total weight of the beaker 2. record the weight of an Alka Seltzer tablet 3. drop the tablet into the beaker, carefully swirl the cup to ensure that the tablet dissolves completely 4. record the weight of the beaker containing water and the dissolved substances once the bubbling ceases 5. wash and rinse the beaker with water 6. calculate the mass of carbon dioxide generated 7. calculate the mass of NaHCO3 reacted 8. calculate the percent by mass of the reacted NaHCO3 in the tablet repeat the experiments with: (see chart below) o 5 mL vinegar + 30 mL deionized water o 10 mL vinegar + 25 mL deionized water o 15 mL vinegar + 20 mL deionized water o 20 mL vinegar + 15 mL deionized water o 25 mL vinegar + 10 mL deionized water o 30 mL vinegar + 5 mL deionized water o 35 mL vinegar + 0 mL deionized water IV. Safety and Hazards Do not eat or taste the vinegar and Alka Seltzer tablets. All solutions can be diluted with water and disposed of down the sink after the experiment. V. Table of Data Run 1 Vol vinegar, mL 0 Vol water, mL 35 Wght of beaker & solution, g Wght of Alka Seltzer tablet, g Wght of beaker after bubbling, g Wght loss of beaker, g Mass of NaHCO3 reacted, g % by mass of NaHCO3 in Alka Seltzer tablet Run 2 5 30 Run 3 10 25 Run 4 15 20 Run 5 20 15 Run 6 25 10 Run 7 30 5 Run 8 35 0 2 VI. Plot of the percent by mass of the reacted NaHCO3 in a tablet versus the volume of vinegar used Use Microsoft Excel to plot the percent by mass of the reacted NaHCO3 in a tablet versus the volume of vinegar used. VII. Questions 1. What is the chemical equation corresponding to the reaction that causes the bubbling when an Alka-Seltzer tablet is dropped into water? 2. How does this equation illustrate principles of stoichiometry? 3. How do you interpret the graph of mass of the reacted NaHCO3 in a tablet versus the volume of vinegar in terms of the concept a of limiting reactant? VIII. References Chen, Y.-H., J.-F. Yuang (2002) Alka-Seltzer fizzing—determination of percent by mass of NaHCO3 in Alka-Seltzer tablets: an undergraduate general chemistry experiment. J. Chem. Educ. 79(7) 848-850. 3