Alka Seltzer and the Ideal Gas Law Lab: Supplementary Information
advertisement

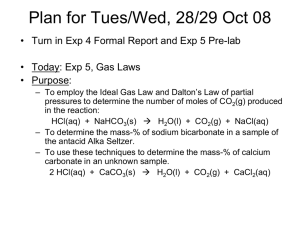
Alka Seltzer and the Ideal Gas Law Lab: Supplementary Information To be eligible for an A on the lab, you must determine the percent yield of this experiment. You will use the following information to find percent yield and must add all calculations to your lab write up as question 9. You may type your answers into this sheet and attach it to your lab handout or you can write all the calculations directly onto your lab handout. Ingredients in each tablet: 1000 mg anhydrous citric acid (H3C6H5O7) 325 mg aspirin (C9H8O4) 1916 mg sodium bicarbonate (NaHCO3) Basic reaction: HCO3-(aq) + H+(aq) => H2O(g) + CO2(g) While the Alka Seltzer tablet does contain aspirin, but this does not react to form a gas, so we will ignore it. The sodium bicarbonate does react to form a gas. The sodium (Na+) dissociates from the bicarbonate (HCO3-). We’ll ignore the sodium since it doesn’t actually end up doing anything (we’ll save the explanation of why for the acid/base chapter). The carbon and oxygen in the bicarbonate will eventually form the CO2. However, the bicarbonate must first react with the H+ that comes off of the citric acid (again, acid/base chapter). Stoichiometry: If we know the amount of either sodium bicarbonate or citric acid, then we can predict the amount of CO2 gas that should form. This will be our theoretical yield. Before you can use stoichiometry, you need to find out how much of the sodium bicarbonate should be in YOUR Alka seltzer tablet. Mass percent of the sodium bicarbonate (NaHCO3) in Alka Seltzer: 1916 mg NaHCO3 / (1000 mg H3C6H5O7 + 325 mg C9H8O4 + 1916 mg NaHCO3)= 0.59117556 0.59117556 x 100%= 59.1% NaHCO3 in an Alka Seltzer tablet Finding the mass of sodium bicarbonate in YOUR Alka Seltzer tablet: Find the total mass of your Alka Selzer tablet (put it on the scale). We’ll call it “X g Alka Seltzer” for now, but you’ll replace X with the real mass when you do this calculation. X g Alka seltzer x (59.1 g NaHCO3/100 g Alka Seltzer)= ___ g NaHCO3 Back to the stoichiometry: ____ g NaHCO3 x (1 mol NaHCO3/ 84.01 g NaHCO3) x (1 mol CO2/ 1 mol NaHCO3) x (44.01 g CO2/ 1 mol CO2)= ____ g CO2 Theoretical yield of CO2: That’s the answer to the previous question Your percent yield of CO2: You have your theoretical yield of CO2. It’s up to you to find your actual yield of CO2 by doing the experiment. Once you have that you can plug the numbers into the following equation: Percent yield= (Actual/Theoretical) x 100%