EXPERIMENT 1 The Equation for a Reaction
advertisement

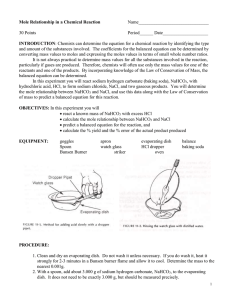
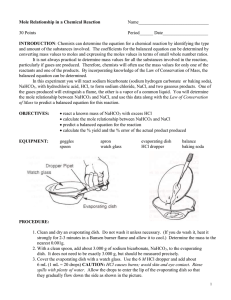
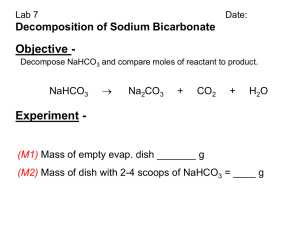
EXPERIMENT 1 The Equation for a Reaction The purpose of the experiment is: To determine the molar ratio between sodium hydrogen carbonate and hydrochloric acid when they react. To write an equation for the reaction. INTRODUCTION A chemical equation describes what happens during a chemical reaction. The equation identifies : Reactants (starting materials) . Products (resulting substances). Molecular Formulas of substances. Physical states the substances (solid, liquid, gas or aqueous) Molar quantities of each substance. There are essentially three steps to write a balance equation: 1. Write an unbalanced equation. • Chemical formulas of reactants are listed on the left hand side of the equation. • Products are listed on the right hand side of the equation. • Reactants and products are separated by putting an arrow between them to show the direction of the reaction. Reactions at equilibrium will have arrows facing both directions. 2. Balance the equation. • Apply the Law of Conservation of Mass to get the same number of atoms, or same number of moles, of every element on each side of the equation. Tip: Start by balancing an element that appears in only once in reactants and products. Once the first element is balanced, proceed to balance the second, and so on, until all elements are balanced. • Balance the elements in both side by placing numbers as coefficients where appropriate. 3. Indicate the physical states of the reactants and products. ◦ Use (g) for gaseous substances. ◦ Use (s) for solids. ◦ Use (l) for liquids. ◦ Use (aq) for dissolved substance in water. ◦ Write the appropriate physical states using the above symbols following the molecular formulas. CHEMICALS USED 2 M HCl Sodium hydrogen carbonate, NaHCO3(s) Lime water, Ca(OH)2(aq) Dilute silver nitrate APPARTUS Conical flask Test tube Hot plate Dish Delivery tube Procedure : PART 1: Study the behaviour of CO2 gas A . Reactions Between Carbon Dioxide and Limewater: limewater can be used to detect the presence of carbon dioxide because limewater reacts with carbon dioxide to produce a white precipitate of calcium carbonate: Set up the apparatus as shown . a1 . Half- full the test tube with lime water Ca(OH)2 Clear solution of lime water a2. Place about 2 g NaHCO3 (S) and 3 cm3 of 2M HCl (aq) in a small conical flask and stopper it immediately. 2 g NaHCO3 3 cm3 of 2M HCl Observation: When CO2(g) is bubbled through colorless limewater Ca(OH)2(aq) the limewater turns milky due to the formation of a calcium carbonate (CaCO3(s)) precipitate according to the following equations: NaHCO3(s) + HCl(aq) CO2(g) + Ca (OH)2 NaCl(aq) + CO2 (g) CaCO3(s) +H2O B.Effect of CO2 gas on a lighted splint Repeat step a2 and collect the evolved gas in an empty test tube , and then test with a lighted splint 2 g NaHCO3 3 cm3 of 2M HCl PART 2: Mole ratio between NaHCO3 & NaCl and Salt test: 1. Weigh an empty clean dish 2. Weigh about 0.6 g NaHCO3 3. Place the evaporating dish on a hot plate and carefully (drop wise) add a dilute hydrochloric acid (2 M). When all of the bicarbonate has reacted, begin to heat the dish. 4. Heat to dryness. 5. Let the dish to cool to room temperature, weigh and record the mass Chloride test: Dissolve some of the residue in a test tube using distilled water. Add few drops of silver nitrate to test for Cl- ions. Record your observations and write an equation) When AgNO3 solution is added to the residue, a white precipitate of AgCl is formed . NaCl(s) + AgNO3(aq) AgCl(s) + NaNO3(aq) Data Sheet: ] Mass of empty evaporating dish, m1= Mass of evaporating dish + NaHCO3, m2 = Mass of NaHCO3, mNaHCO3 = m2 – m1 = Mass of evaporating dish+residue after heating,m3= Mass of residue, mNaCl = m3 – m1 = Molar mass of NaHCO3, MMNaHCO3 = Moles of NaHCO3 , nNaHCO3 = mNaHCO3 /MMNaHCO3 Molar Mass of Nacl residue, MMNaCl = Moles of NaCl, nNaCl = mNacl/MMNaCl = Mole Ratio of NaHCO3 : NaCl = The balanced equation : NaHCO3(s) + HCl(aq) 12.Test of Cl- : --------(aq) + ------(g)