Molecular Physics
advertisement
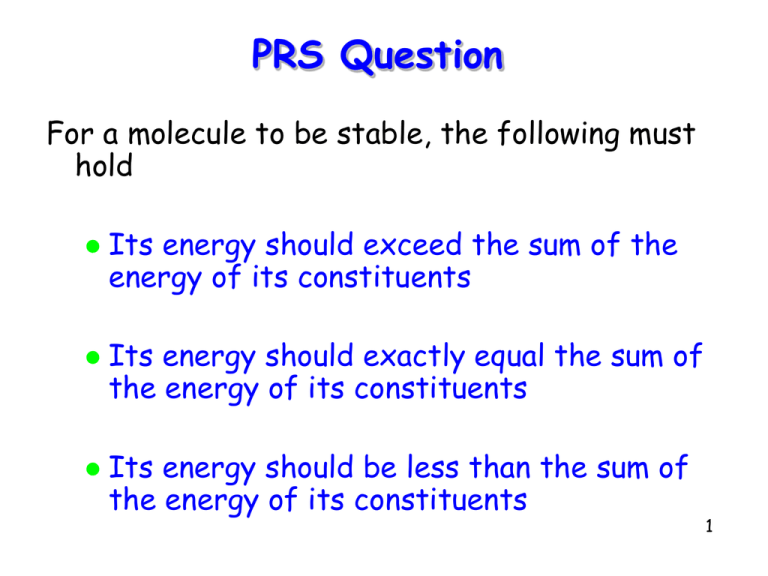
PRS Question For a molecule to be stable, the following must hold Its energy should exceed the sum of the energy of its constituents Its energy should exactly equal the sum of the energy of its constituents Its energy should be less than the sum of the energy of its constituents 1 Molecular Structure 2 2 Topics Recap Exclusion Principle Repulsion The Covalent Bond H2 He2 Summary 3 Recap A molecule, such as KCl, forms only if the following holds Energy(molecule) < ∑i Energy(atomi) that is, the total energy of the molecule is less than the total energy of its constituent atoms when they are widely separated 4 Recap For example, the net total energy of the K+ and Cl- ions, after the transfer of the 4s electron from K to the unoccupied 3p state in Cl is 0.72 eV. This is not low enough to cause the ions to bind. However, if they are brought within 2.8 nm of each other their total energy falls below zero and the ions bind K+ 2.8 nm Cl5 Exclusion Principle Repulsion As atoms are brought closer and closer together, their wavefunctions overlap more and more But, since only one electron can occupy a given state, some electrons will be forced to occupy higher (empty) energy levels Another way to say this is that electrons in the same spin state tend to repel each other because of the Pauli exclusion principle 6 Exclusion Principle Repulsion This tendency of electrons to stay away from each other increases the energy of a system of atoms as they are brought together, while the electrostatic energy decreases Consequently, there will be some separation between the atoms, called the equilibrium position, r0, for which the energy of the molecule is a minimum 7 Exclusion Principle Repulsion Example: For molecules like KCl, the potential energy is a sum of the electrostatic potential, the exclusion principle energy and the net ionization energy: 2 e U (r ) k Eex Eion r The energy needed for the reaction K+Cl- -> K + Cl is called the dissociation energy, Ed 8 Exclusion Principle Repulsion r0 = 0.27 nm, Ed = 4.4 eV 9 10 The Covalent Bond To make an H2 molecule, we could first try to transfer an electron and create the ions H+ and H- and then bring them together The net ionization energy is 12 eV It turns out there is no distance at which the loss in potential energy is greater than 12 eV So the H2 molecule cannot form this way 11 The Covalent Bond The binding of two hydrogen atoms to form H2 is a pure quantum effect: the sharing of the two electrons by the hydrogen atoms To see how this works, we consider the quantum mechanics of two 1-D square wells of finite depth and width L 12 The Covalent Bond Wells far apart, wave functions do not overlap Energies are identical for both states 13 The Covalent Bond When close, the wave functions look as shown Then Energy(yA) is now greater than Energy(yS) 14 The Covalent Bond – H2 Symmetric Anti-symmetric When far apart the total energy of the two hydrogen atoms, each in their 1s (ground) state, is -13.6 eV + -13.6 eV = -27.2 eV. Unstable configuration Stable configuration In other words, the energies of the symmetric and anti-symmetric states are the same. The ground state with energy -27.2 is said to be doubly degenerate 15 The Covalent Bond – H2 When the atoms are brought close together, however, the energies of the symmetric and Symmetric anti-symmetric states areAnti-symmetric no longer identical Stable configuration Unstable configuration 16 The Covalent Bond – H2 The symmetric state, in which electrons have a high probability of being between the protons, Symmetric has lower energy than theAnti-symmetric anti-symmetric state. The symmetric state is called a bonding orbital The other state is called an anti-bonding orbital Stable configuration Unstable configuration 17 The Covalent Bond – H2 However, for H2 to form the symmetric state must be able to accommodate both electrons. Anti-symmetric Symmetric This can happen because the electron spins are anti-parallel, that is, have total spin S = 0. H2 is said to be s-bonded Stable configuration Unstable configuration 18 The Covalent Bond – H2 The energy diagram of H2 as a function of separation r This kind of bonding is called a covalent bond 19 The Covalent Bond – He2 He has no valence electrons that can be shared. Consequently, 2 electrons will occupy the 1s bonding orbital, which is then a saturated bond, while the other 2 electrons are forced to occupy the higher energy anti-bonding orbital Since the combined energy of the bonding and anti-bonding orbitals exceeds that of 2 widely separated He atoms, the He2 molecule does not form 20 Summary The exclusion principle induces an additional repulsive force between electrons, which can balance the electrostatic forces yielding a separation between atoms for which the total energy is a minimum As two atoms are brought together, bonding and anti-bonding orbitals form with the former having the lower energy. Whether or not a molecule forms depends on the total energy of the occupied orbitals. 21