Formulas and Percent Composition
advertisement

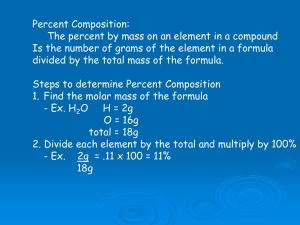
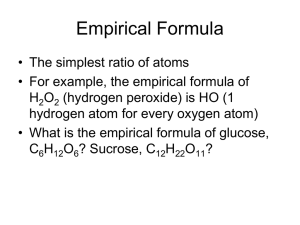
Formulas and Percent Composition Finding the Mystery Formulas Using Analytical Data Scientist synthesize new compounds for many uses. Once they make a new product, they must check its identity. One way to carry out a chemical analysis is by providing a percent composition. Example in History In 1962, scientists made a new compound from xenon and fluorine. Before 1962, scientist believed xenon did not form compounds. The scientists did a percent composition of the compound and found it was 63.3% Xe and 36.7 % F, which is the same as that for a formula XeF4. Percent Composition helps identify a substance by comparing ratio of masses (empirical formula). Determining % Composition % Composition = Mass of part of sample Mass of whole sample X 100 *% composition remains consistent regardless of the size of the sample Example % Comp Problem Ex. Find the % composition of Ca, O and H in a Ca(OH)2 sample. Based on a 20.18 g sample of Ca(OH)2, how many grams did the individual elements contribute? Determining Empirical Formulas Data for a percent composition allow you to calculate the simplest ratio among the atoms found in a compound. Empirical Formula is the simplest ratio of atoms in a compound Ammonium Nitrite formula is NH4NO2 Ammonium Nitrite empirical Formula is NH2O Percent Composition To Empirical Formula *Step 1 – change % to grams, assuming a 100 g sample *Step 2 – convert grams to moles *Step 3 – divide all mole values by the smallest mole value to try and get all values into simple whole numbers *Step 4 – if mole values are not whole numbers after step 3, multiply all mole values by the same smallest whole number to achieve this *Step 5 – write the empirical formula of the compound Example Problems A compound is found to have 79.8% carbon and 20.2% hydrogen by mass. What is the empirical formula of this compound? A compound is composed of 58.8% C, and 9.8% H. What is the empirical formula of this compound? More Practice Problems 60.0% C, 13.4% H, 26.6% O. Find the empirical formula. A dead battery is found to contain a compound of 69.9% Mn and 30.4% O. What is the empirical formula? Magnetic iron oxide is 72.4% iron and 27.6% oxygen. What is the empirical formula? Empirical formulas not molecular formulas In determining empirical formulas, the simplest ratio of atoms was determined. This is not necessarily the exact number of atoms in the compound which is known as the molecular formula. Additional information is needed to determine molecular formulas. Molecular Formulas Molecular Formulas are made of single molecules. For many compound the molecular formula is a simple whole number ratio of the empirical formula The molar mass of a molecular formula is equal to the molar mass of a empirical formula multiplied by “n” (a whole number) Process If you divide the experimental molar mass by the molar mass of the empirical formula, you can figure out the value of n needed to scale the empirical formula up to give the molecular formula. n(emperical formula) = molecular formula Example Problem The empirical formula of a compound is CH3N. The mass of the molecular formula is 60.12 g/mol. What is the molecular formula of this compound? 1. 2. 3. Steps To Find The Molecular Formula From The Empirical Formula Find molar mass of the empirical formula Divide the molecular mass given in the problem by the empirical formula mass to get the multiple of how many times larger the molecular mass is compared to the empirical mass (n). Use the multiple you found in step 2 to write your molecular formula. This must mean that the # of atoms of the empirical formula must be multiplied by this multiple n(empirical formula) More Example Problems Molar mass of 78 g/mol and empirical formula of CH, what is its molecular formula? A brown gas on the cabinet to your right has the empirical formula of NO2. Its experimental molar mass is 46g/mol. What is its molecular formula? The lichens presents on the bark of trees contain a substance called succinic acid. The organic acid is also present in fungi, which is used to make dyes and pefumes. Percent composition is 40.68% C, 5.08% H, 54.24 % O. Its molecular mass is 118.8 g/mol What is its empirical and molecular formulas?