Chapter 7 Section 3
advertisement

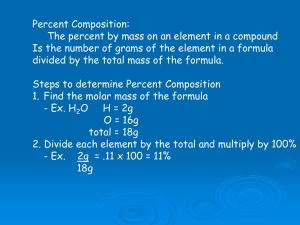
Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Definitions: Percent Composition: the percentage by mass of each element in a compound Empirical Formula a chemical formula that shows the composition of a compound in terms of the relative numbers and kinds of atoms in the simplest ratio Molecular Formula a chemical formula that shows the number and kinds of atoms in a molecule, but not the arrangement of the atoms Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Percent Composition Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition How to calculate the percent composition of a compound. 1. Calculate the mass of each element in the compound. For example if the compound contains 3 oxygen atoms the mass of the oxygen in the compound is 16.0 x 3 = 48 2. Calculate the gram molar mass of the whole compound. For example if the compound was CaSO3, the total mass would be 40.1 from calcium + 32.0 from sulfur and 48.0 from oxygen. The total would be 40.1 + 32.0 + 48.0 for A total mass of 120.1. 3. Follow the formula on your Reference Table: Mass of the part is step 1, mass of the whole is step 2. Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Practice you percent composition skills 1. Calculate the percent composition of sodium chloride, NaCl Na – 39.7% Cl- 60.3 % 2. Calculate the percent composition of silver nitrate,AgNO3 Ag - 63.5% N - 8.2% O - 28.3% 3. Calculate the percent composition of magnesium hydroxide, Mg(OH)2 Mg – 41.7% O – 54.9% H - 3.4% 4. What is the mass percentage of water in the hydrate CuSO4• 5 H2O Water is 36.1% of the compound Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Homework Page 860 #47, #48 and #49 Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Difference between empirical formula and molecular formula Empirical Formula: Simplest ratio of elements Molecular Formula: “True formula” Does not have to be simplest ratio C6H12O6 Molecular Reduced by factor of 6 CH2O Empirical H2 O Both empirical and molecular Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Calculating Empirical Formula 1. Set up the problem by each element 2. divide each element quantity by its gram molar mass from the periodic table 3. Look at each answer and divide the smallest answer into each elements answer. 4. This will produce the ratio of each element in the empirical formula 5. If ratio is not whole numbers increase all answers by a factor to create whole numbers 6. Write empirical formula Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition A compound is found to contain 54.5% carbon, 9.1% hydrogen, and 36.4% oxygen. Determine the simplest formula. Carbon Hydrogen Oxygen 54.5 12 9.1 1.0 36.4 16.0 4.54 2.27 9.1 2.27 2.27 2.27 2 4 1 C2H4O Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Find the empirical formula of a compound found to contain 26.56% potassium, 35.41% chromium, and the remainder oxygen. Potassium Chromium Oxygen 26.56 39.1 35.41 52.0 38.03 16.0 0.68 0.68 0.68 0.68 2.34 0.68 1.0 1.0 3.5 Increase by factor of 2 to make all whole numbers K2Cr2O7 Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Homework page 861 #69 - #73 Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Calculating Molecular Formula 1. Calculate the empirical formula if not given. 2. Calculate the empirical formulas gram formula mass 3. Divide the molecular formulas gram formula mass by the molecular formulas gram formula mass. This will give you a factor number that you must increase the ratio of the empirical formula by. Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Determine the molecular formula of a compound with an empirical formula of NH2 and a formula mass of 32.06 amu. NH2 gram formula mass is 16.0 amu Molecular formulas gram formula mass is 32.06 amu 32.06 16.0 = 2 You must increase the ratio of NH2 by a factor of 2 N2H4 Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition Chapter 7 – The Mole and Chemical Composition Sec 3 - Formulas and Percentage Composition