CH 10 Notes Part Two
advertisement
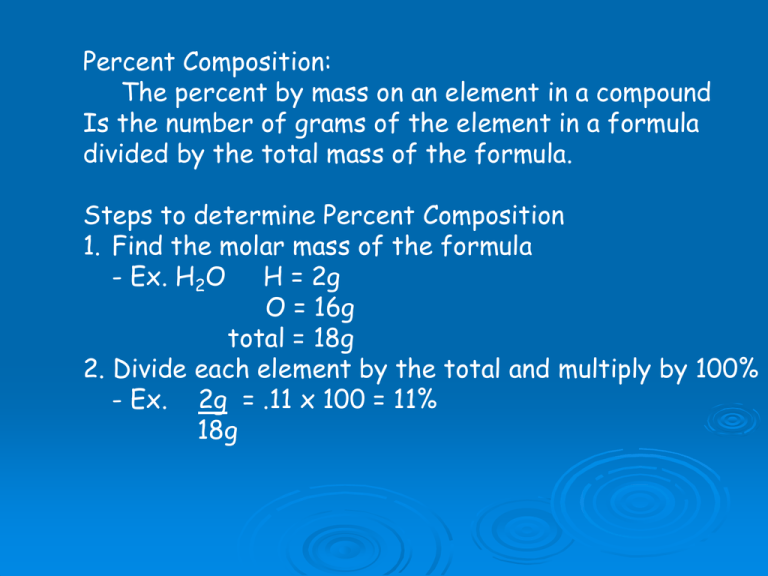
Percent Composition: The percent by mass on an element in a compound Is the number of grams of the element in a formula divided by the total mass of the formula. Steps to determine Percent Composition 1. Find the molar mass of the formula - Ex. H2O H = 2g O = 16g total = 18g 2. Divide each element by the total and multiply by 100% - Ex. 2g = .11 x 100 = 11% 18g Empirical Formula: The Empirical Formula of a compound shows the smallest whole number ratio of the atoms in the compound Which of the following are empirical formulas? Sodium peroxide, NaO Terephthalic acid, C8H6O4 Phenobarbital, C12H12N2O3 Now that you can identify an empirical formula, let us learn to calculate one. There are three steps in calculating an empirical formula Let us try an example Ex. Calculate the empirical formula of a compound that contains 67.6% Hg, 10.8% S, 21.6%O 1. Change the percentage to grams and convert the mass to moles for each element in the formula 67.6g Hg x 1 mole Hg = .34 mol Hg 201g Hg 10.8g S x 1 mole S = .34 mol S 32g S 21.6g O x 1 mole O = 1.4 mol O 16g O 2. Find the lowest molar value and divide it into itself and all the other molar values 67.6g Hg x 1 mole Hg = .34/.34 = 1 201g Hg 10.8g S x 1 mole S = .34/.34 = 1 32g S 21.6g O x 1 mole O = 1.4/.34 = 4 16g O 3. The number just calculated becomes the subscript of that atom in the formula. 67.6g Hg x 1 mole Hg = .34/.34 = 1 = Hg1 201g Hg 10.8g S x 1 mole S = .34/.34 = 1 = S1 32g S 21.6g O x 1 mole O = 1.4/.34 = 4 = O4 16g O Therefore, the empirical formula is HgSO4 Look familiar? Mecuric Sulfate! So lets review List the steps for percent composition. Try the sample problems on pages 306,307 List the steps for empirical formula calculations. Try the sample problems on page 310 Work on the handouts – if you lost them they are on the web-page Now let us learn how to calculate molecular formulas: -The molecular formula of a compound is either the same as its empirical formula or a aimple whole number multiple of the empirical formula. Ex. CH empirical, C2H4 molecular Remember an empirical formula can not be reduced but a molecular formula can be reduced. Determining Molecular Formulas: 1. Calculate the empirical formula 2. Calculate the molar mass of the empirical formula 3. Divide the mass of the molecular formula by the molar mass of the empirical formula 4. Use that value to multiply with each subscript of the empirical formula. Remember our example of an empirical formula for HgSO4? Well the sum of this formula is 297g what would the Molecular formula be if the molecular mass was 594g? 594g/297g = 2 therefore Hg2(SO4)2 becomes Hg2(SO4)2