5. Average Atomic Mass
advertisement
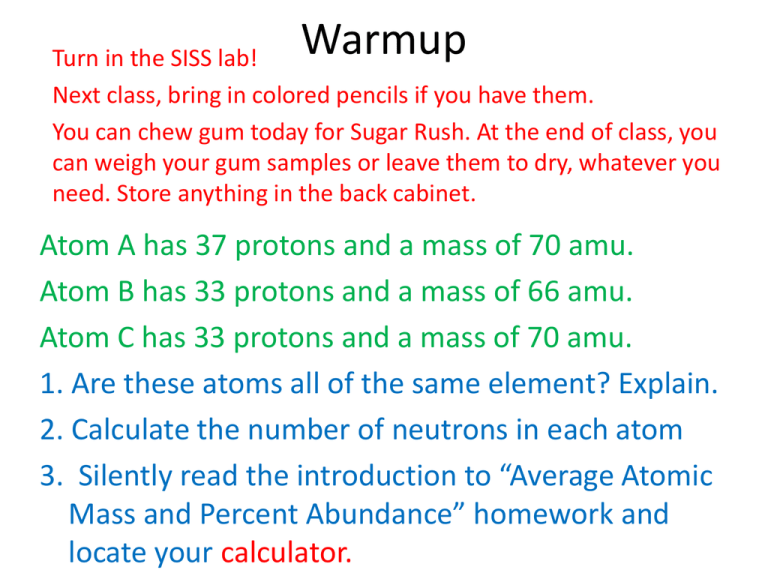
Warmup Turn in the SISS lab! Next class, bring in colored pencils if you have them. You can chew gum today for Sugar Rush. At the end of class, you can weigh your gum samples or leave them to dry, whatever you need. Store anything in the back cabinet. Atom A has 37 protons and a mass of 70 amu. Atom B has 33 protons and a mass of 66 amu. Atom C has 33 protons and a mass of 70 amu. 1. Are these atoms all of the same element? Explain. 2. Calculate the number of neutrons in each atom 3. Silently read the introduction to “Average Atomic Mass and Percent Abundance” homework and locate your calculator. Average Atomic Mass 1. The atomic mass of copper 63, is 62.93 amu, and has an abundance of 69.09%. Approximately 30.91% of copper atoms exist as copper 65, having an atomic mass of 64.9278 amu. The atomic mass shown on the periodic table for copper is 63.55 amu. How did scientists arrive at this value? average atomic mass for an element % abundance of isotope 1 (in decimal form) = average atomic mass for Cu = average atomic mass for Cu a.a.mCu 0.6909 atomic mass of isotope 1 x x 62.93 43.478337 = = 63.55 amu % abundance of isotope 2 (in decimal form) + + + 0.3091 atomic mass of isotope 2 x x 20.06918298 64.9278 2. Naturally occurring sulfur consists of four isotopes, S-32 (95.0%), S-33 (0.76%), S-34 (4.22%), and S-36 (0.014%). Using these data, calculate the atomic weight of naturally occurring sulfur. The masses of the isotopes are: S-32: 31.97amu S-33: 32.97amu S-34: 33.97amu S-36: 35.97amu % abund. S-32 0.950 x x 31.97 amu 31.97 amu 30.3715 + + % abund. S-33 x 0.0076 + 32.97 amu x + % abund. S-34 32.97 + 0.0422 amu 0.250572 + 33.97 amu x x 1.433534 + 33.97 amu + 32.06 amu (very close to the PT value) + % abund. S-36 x 0.00014 0.0050358 35.97 amu 35.97 amu x = = = 3. Rubidium has only 2 known stable isotopes. Calculate the % abundance of each isotope 85Rb = 84.9118 amu 87Rb = 86.9092 amu % abundance of isotope 1 (in decimal form) x atomic mass of isotope 1 x x 84.9118 amu % abundance of isotope 2 (in decimal form) + + y atomic mass of isotope 2 x x 86.9092 amu average atomic mass of Rb = = 85.47 amu y=1-x x x 84.9118 amu + 1-x x 86.9092 amu = 85.47 amu 84.9118x + 1(86.9092) – x(86.9092) = 85.47 84.9118x + 86.9092 – 86.9092x = 85.47 x = 0.721, y = 0.279 -1.9974 x = -1.4392 -1.9974 -1.9974