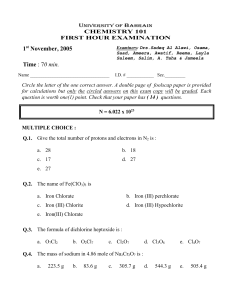
The formula for oxalic acid is (COOH)2. How many atoms does each
advertisement
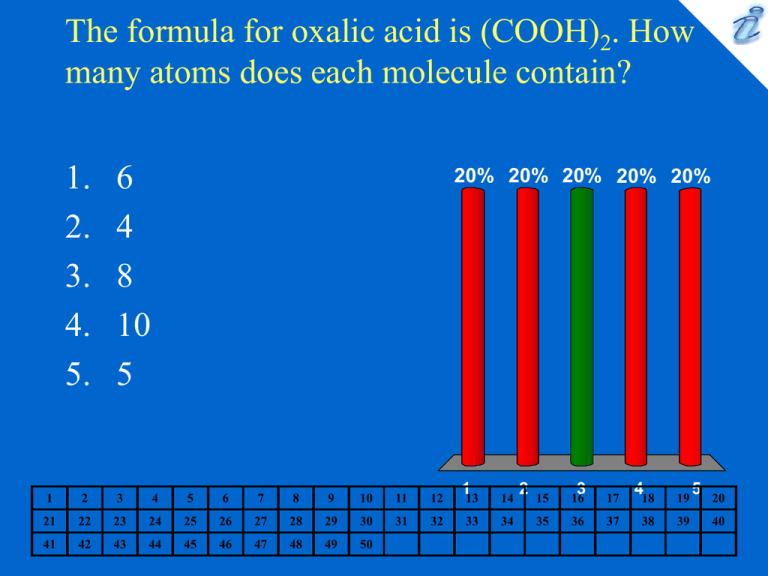
The formula for oxalic acid is (COOH)2. How many atoms does each molecule contain? 1. 2. 3. 4. 5. 6 4 8 10 5 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What is the molecular formula for acetic acid? 1. 2. 3. 4. 5. CH3COOH CH3COCH3 CH3CH2OH CH3CH2CO2H CH3CH2OCH2CH3 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What is the formula for iron(III) oxide? 1. 2. 3. 4. 5. FeO3 FeO2 Fe3O2 Fe2O3 Fe3O 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Choose the name-formula pair that does not correctly match. 1. magnesium phosphate Mg3(PO4)2 2. ferrous sulfite FeSO3 3. silver carbonate AgCO3 4. potassium fluoride KF 5. cupric bromide CuBr2 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 If 6.653 g of calcium combines exactly with 26.53 g of bromine and 12.29 g of zinc combines exactly with 30.04 g of bromine, what is the mass ratio of one atom of calcium to one atom of zinc? 1. 2. 3. 4. 5. 1.68 0.613 0.541 1.85 0.133 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Calculate the number of atoms in 40.5 g of aluminum. 1. 2. 3. 4. 5. 900 2.5 x 10-24 9.0 x 1023 6.6 x 1026 1.8 x 10-21 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What is the formula weight of Fe(NH4)2(SO4)2•6H2O? 1. 2. 3. 4. 5. 392 amu 384 amu 412 amu 376 amu 436 amu 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 How many atoms of hydrogen are there in 88 g of C6H6? 1. 2. 3. 4. 5. 3.6 x 1023 6.8 x 1022 2.6 x 1023 4.1 x 1024 5.3 x 1023 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 How many hydrogen atoms are present in 7.50 g of (NH4)2Fe(SO4)2•6H2O? 1. 2. 3. 4. 5. 5.16 x 1022 2.30 x 1023 1.15 x 1022 4.65 x 1023 3.35 x 1024 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 Calculate the percent composition of FeSO4. 1. 2. 3. 4. 5. %Fe = 27.9%, %S = 24.1, %O = 48.0% %Fe = 16.2% , %S = 27.9%, %O = 55.8% %Fe = 33.2% , S = 9.5% , %O = 57.2% %Fe = 36.8%, S = 21.1%, %O = 42.1% %Fe = 25.1%, %S = 21.6% , %O = 53.2% 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What would be the empirical formula for a compound containing 33.36% calcium, 26.69% sulfur, and 39.95% oxygen? 1. 2. 3. 4. 5. CaSO4 CaSO2 CaSO CaSO3 Ca2S2O5 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 The complete combustion of a hydrocarbon produced 352 mg of CO2 and 216 mg of H2O. What is the simplest formula of this hydrocarbon? 1. 2. 3. 4. 5. CH CH C2H3 CH3 C3H2 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 The complete combustion of a hydrocarbon produced 26.4 g of CO2 and 5.40 g of H2O. Another experiment determined the molecular weight of this hydrocarbon to be approximately 52 g/mol. What is its molecular formula? 1. 2. 3. 4. 5. C2H4 CH C4H4 C2H2 CH4 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What mass of lead is present in 5.05 kg of mendipite, PbCl2•2PbO? 1. 2. 3. 4. 5. 2.89 kg 4.05 kg 5.05 kg 3.68 kg 4.33 kg 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40 What mass of fluorine is contained in 2.00 tons of cryolite that is 42.0% pure Na3A1F6? (No other compounds containing fluorine are present.) 1. 2. 3. 4. 5. 912 lb 832 lb 456 lb 304 lb 152 lb 20% 20% 20% 20% 20% 1 2 3 4 5 6 7 8 9 10 11 12 113 14 21 22 23 24 25 26 27 28 29 30 31 32 33 34 41 42 43 44 45 46 47 48 49 50 2 15 16 3 17 418 19 35 36 37 38 39 5 20 40