Mass Defect, Binding Energy, and Nuclear Reactions
advertisement

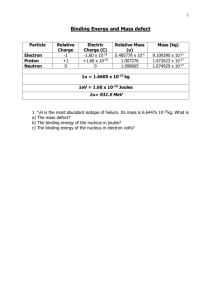
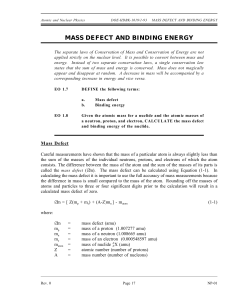
Do Now (5/1/14): • Pick up a note sheet from the back on your way in • What particles exist in an atom? (on a new Do Now sheet) • Rank those particles in order of most massive to least massive (if you are not sure, guess) Mass Defect, Binding Energy, and Nuclear Reactions 5/1/14 Mass Defect: • The difference between the sum of the mass of the individual nucleon (proton or neutron) and the actual mass. m matom Z m m p e (A Z)m n Example: • Find the mass defect of a copper-63 nucleus if the actual mass of a copper-63 nucleus is 62.91367 amu Find the composition of the copper-63 nucleus and determine the combined mass of its components. Copper has 29 protons and copper-63 also has (63 - 29) 34 neutrons. The mass of a proton is 1.00728 amu and a neutron is 1.00867 amu. The combined mass is calculated: 29 protons(1.00728 amu/proton) + 34 neutrons(1.00867 amu/neutron) or 63.50590 amu Example: • Find the mass defect of a copper-63 nucleus if the actual mass of a copper-63 nucleus is 62.91367 amu • Calculate the mass defect. • Dm = 63.50590 amu - 62.91367 amu = 0.59223 amu Binding Energy E binding (m assdefect(inu))(931.49MeV /u) • The energy equivalent of the mass defect; it is always negative It is the minimum amount of energy needed to break the nucleus into its component nucleons. Binding Energy of Alpha Particle For the alpha particle Δm= 0.0304 u which gives a binding energy of 28.3 MeV It Takes a Lot More Energy to Split a Nucleus Than to Ionize an Atom Practice: Work on the Mass Defect worksheet. Finish all mass defect and binding energy problems by the end of class for extra credit! Do Now (5/2/14): 1. What is the mass defect of an alpha particle if its mass is 4.00153u? 2. What is the binding energy of an alpha particle? Nuclear Notation Balancing Nuclear Decay Equations 92U 238 --------> 90Th234 + 2He4 Subscripts are "proton numbers" Superscripts are "nucleon numbers" Proton and nucleon counts must be the same: 92 = 90 + 2 238 = 234 + 4 Decay Sequence Alpha decay sequence: 235 4 U 92 231 He + 2 209 4 Po 84 Th 90 205 He + 2 Pb 82 Decay Sequence Beta decay sequence: 14 14 C 6 e N + 7 228 Ra 88 0 -1 228 0 e Ac + 89 -1 Practice: • Use the rest of class to work on “Mass Defect and Binding Energy” and “Nuclear Reactions.”