Atomic Structure
advertisement
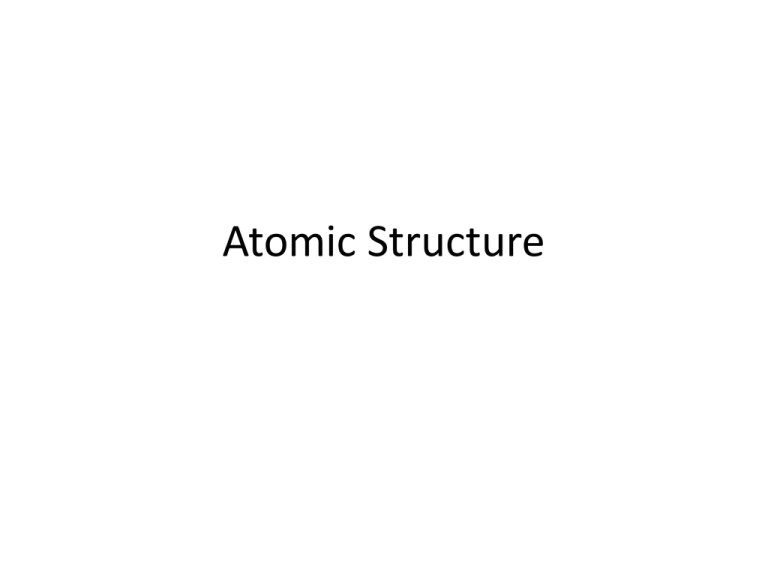
Atomic Structure Atomic Number • Definition: Equals the number of protons – Identifies the element • # protons = # electrons in a neutral atom (an atom without a charge) Isotopes & Mass Number • Isotopes = atoms with the same # of protons, but different numbers of neutrons Ex: Isotopes 3 isotopes of hydrogen: 1. Hydrogen-1; mass # = 1; 0 neutrons 2. Hydrogen-2 (deuterium); mass # = 2; 1 neutron 3. Hydrogen-3 (tritium); mass # = 3; 2 neutrons There are two different ways to write chemical symbols for isotopes: 1. Write the mass number after the element’s name (gold-197) 2. Use the symbol, a superscript for mass #, and a subscript for atomic # Mass Number = protons + neutrons • To calculate # neutrons = mass # - atomic # • Use shorthand writing of atom’s composition • Superscript = mass # • Subscript = atomic # Practice 1 Practice 2 Practice 3 a) Three isotopes of sulfur are sulfur-32, sulfur33, and sulfur-34. Write the complete symbol for each isotope, including the atomic number and the mass number. b) How many neutrons, protons, and electrons are in Na+ with a mass number of 24? What is its atomic number? Atomic Mass • Weighted atomic mass of all the isotopes of that element – that is why it is a decimal, not an integer • It reflects mass and relative abundance of the isotopes as they occur naturally AMU – the mass of an atom • Amu’s are used to define the weight of a single atom or isotope • How measured: Mass spectrometer – determines masses of atoms • Carbon-12 was set to 12 atomic mass units (amu’s) – Used to define masses of atoms Mass Number and Atomic Mass Carbon-12 = 6 protons and 6 neutrons, – mass # = 12, – atomic mass = 12 amu, (atomic # = 6) • 1 proton = 1 amu • 1 neutron = 1 amu • Mass number = Atomic Mass FOR A SINGLE ATOM ONLY • The defined mass of each elements is actually a weighted average so it is NOT a whole number. Average Atomic Mass: Uses a weighted average to account for mass and relative amounts • Most elements are present as a mixture of 2+ isotopes • Not all isotopes are present in equal amount (percent abundance) • Ex – hydrogen Mass defect: the mass of any nucleus is less than the sum of the separate masses of its protons and neutrons. How can the actual mass be less than the mass number? Can atoms lose mass? • E=mc2... Some of the mass of the protons and neutrons get converted to energy when they come together in the nucleus Calculating average atomic mass • Atomic mass = (Mass isotope A * natural abundance of A) + (Mass isotope B * natural abundance of B) + … • You must know the following in order to calculate the atomic mass of an element: – # of stable isotopes – mass of each isotope – % abundance of each isotope Chlorine: Example 1 • Use the data from the previous slide on chlorine to determine the average atomic mass. Magnesium Example Mass Number % Abundance 24 78.99 25 10.00 26 11.01 Calculating Relative Abundance from Average Atomic Mass • The element Quahog has two isotopes: Quahog-35 and Quahog-40. – If the atomic mass of Quahog is 39.00 amu, what is the relative abundance of each isotope? • The sum of the relative abundance must equal 1. • So assign X and 1-X as the relative abundance of each and solve.