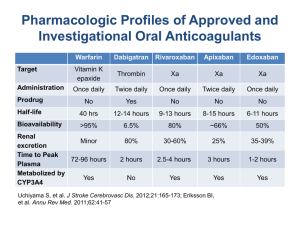
ROCKET AF Results - Duke Clinical Research Institute
advertisement

Rivaroxaban Once-daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation Kenneth W. Mahaffey, MD and Keith AA Fox, MB ChB on behalf of the ROCKET AF Investigators Relevant Financial Relationships Kenneth W. Mahaffey, MD Research Grants: AstraZeneca, Bayer, BI, BMS, Eli Lilly, J&J, Merck, Novartis, Portola, Regado, Sanofi-Aventis, The Medicines Company Consulting Fees: AstraZeneca, Bayer, BI, BMS, Eli Lilly, J&J, Merck, Novartis, Sanofi-Aventis No stock ownership http://www.dcri.duke.edu/research/coi.jsp Keith AA Fox, MB ChB Research Grants: Bayer, Eli Lilly, J&J, Sanofi-Aventis Consulting Fees: Bayer, Eli Lilly, J&J, Sanofi-Aventis No stock ownership Background Rivaroxaban Direct, specific, competitive factor Xa inhibitor TF/VIIa X IX Half-life 5-13 hours VIIIa Clearance : Rivaroxaban Va 1/3 direct renal excretion 2/3 metabolism via CYP 450 enzymes Xa Oral, once daily dosing without need for coagulation monitoring Studied in >25,000 patients in post-op, DVT, PE and ACS patients IXa II IIa Fibrinogen Fibrin Adapted from Weitz et al, 2005; 2008 Risk Factors Study Design Atrial Fibrillation Rivaroxaban 20 mg daily 15 mg for Cr Cl 30-49 ml/min Randomize Double Blind / Double Dummy (n ~ 14,000) • CHF • Hypertension At least 2 or 3 required* • Age 75 • Diabetes OR • Stroke, TIA or Systemic embolus Warfarin INR target - 2.5 (2.0-3.0 inclusive) Monthly Monitoring Adherence to standard of care guidelines Primary Endpoint: Stroke or non-CNS Systemic Embolism * Enrollment of patients without prior Stroke, TIA or systemic embolism and only 2 factors capped at 10% Statistical Methodologies Superiority Sample Size Warfarin event rate ~2.3 Type 1 error 0.05 (2-sided) 405 events; >95% power Non-inferiority Inferiority 1.0 Rivaroxaban Better 1.46 Warfarin Better ~14,000 patients Primary Efficacy Evaluation: Stroke or non-CNS Embolism Non-Inferiority: Protocol Compliant on treatment Superiority: On Treatment and then by Intention-to-Treat Primary Safety Evaluation: Major or non-Major Clinically Relevant Bleeding Enrollment 45 countries, 1178 sites, 14,264 patients Canada: 750 United States: 1,932 Mexico: 168 Panama: 0 Venezuela: 20 Colombia: 268 Peru: 84 Brazil: 483 Chile: 287 Argentina: 569 Poland: 528 Finland: 16 Lithuania: 245 Sweden: 28 Hungary: 237 Norway: 49 Romania: 783 Czech Rep: 598 Bulgaria: 678 Russia: 1,292 Denmark: 123 Ukraine: 1,011 U.K.: 159 Netherlands: 161 Belgium: 96 Korea: 204 China: 496 France: 71 Taiwan: 159 Spain: 250 Germany: 530 Hong Kong: 73 India: 269 Switzerland: 7 Thailand: 87 Philippines: 368 Austria: 32 Malaysia: 51 Italy: 139 Singapore: 44 Greece: 29 Turkey: 101 Israel: 189 Australia: 242 South Africa: 247 New Zealand: 116 Study Conduct Rivaroxaban Warfarin 7131 7133 18 18 1693 (23.9%) 1589 (22.4%) 626 620 589 (396, 805) 593 (404, 810) Median (25th, 75th) Follow-up (days) 706 (522, 884) 708 (518, 886) Randomized, n Lost to Follow-up, n Premature Discontinuation, n (%) Withdrew Consent, n Median (25th, 75th) Exposure (days) Baseline Demographics Age (years) Female (%) Rivaroxaban (N=7081) 73 (65, 78) 40 Warfarin (N=7090) 73 (65, 78) 40 Race (%) White Black Asian 83 1 13 83 1 13 Region (%) North America Latin America Asia-Pacific Central Europe Western Europe 19 13 15 38 15 19 13 15 38 15 Creatinine Clearance (ml/min) (%) 30 - <50 50 - ≤80 > 80 21 47 32 21 48 31 Values are median (IQR) Based on Intention-to-Treat Population Baseline Demographics Rivaroxaban (N=7081) Warfarin (N=7090) 3.48 13 43 29 13 2 3.46 13 44 28 12 2 Prior VKA Use (%) 62 63 Congestive Heart Failure (%) 63 62 Hypertension (%) 90 91 Diabetes Mellitus (%) 40 39 Prior Stroke/TIA/Embolism (%) 55 55 Prior Myocardial Infarction (%) 17 18 CHADS2 Score (mean) 2 (%) 3 (%) 4 (%) 5 (%) 6 (%) Based on Intention-to-Treat Population Trial Results Kenneth W. Mahaffey, MD on Behalf of the ROCKET AF Investigators Primary Efficacy Outcome Stroke and non-CNS Embolism Cumulative event rate (%) 6 5 Event Rate Rivaroxaban Warfarin 1.71 2.16 Warfarin 4 Rivaroxaban 3 HR (95% CI): 0.79 (0.66, 0.96) 2 P-value Non-Inferiority: <0.001 1 0 0 No. at risk: Rivaroxaban 6958 Warfarin 7004 120 240 360 480 600 720 840 960 Days from Randomization 6211 6327 5786 5911 5468 5542 Event Rates are per 100 patient-years Based on Protocol Compliant on Treatment Population 4406 4461 3407 3478 2472 2539 1496 1538 634 655 Primary Efficacy Outcome Stroke and non-CNS Embolism Rivaroxaban Warfarin Event Event Rate Rate On Treatment N= 14,171 Rivaroxaban better P-value 1.70 2.15 0.79 (0.65,0.95) 0.015 2.12 2.42 0.88 (0.74,1.03) 0.117 N= 14,143 ITT HR (95% CI) Warfarin better Event Rates are per 100 patient-years Based on Safety on Treatment or Intention-to-Treat thru Site Notification populations Key Secondary Efficacy Outcomes Rivaroxaban Warfarin Event Rate Event Rate HR (95% CI) P-value 3.11 3.63 0.86 (0.74, 0.99) 0.034 Stroke Type Hemorrhagic Ischemic Unknown Type 0.26 1.34 0.06 0.44 1.42 0.10 0.59 (0.37, 0.93) 0.94 (0.75, 1.17) 0.65 (0.25, 1.67) 0.024 0.581 0.366 Non-CNS Embolism 0.04 0.19 0.23 (0.09, 0.61) 0.003 Myocardial Infarction 0.91 1.12 0.81 (0.63, 1.06) 0.121 All Cause Mortality Vascular Non-vascular Unknown Cause 1.87 1.53 0.19 0.15 2.21 1.71 0.30 0.20 0.85 (0.70, 1.02) 0.89 (0.73, 1.10) 0.63 (0.36, 1.08) 0.75 (0.40, 1.41) 0.073 0.289 0.094 0.370 Vascular Death, Stroke, Embolism Event Rates are per 100 patient-years Based on Safety on Treatment Population Key Secondary Efficacy Outcomes Rivaroxaban Warfarin Event Rate Event Rate HR (95% CI) P-value 4.51 4.81 0.94 (0.84, 1.05) 0.265 Stroke Type Hemorrhagic Ischemic Unknown Type 0.26 1.62 0.15 0.44 1.64 0.14 0.58 (0.38, 0.89) 0.99 (0.82, 1.20 1.05 (0.55, 2.01) 0.012 0.916 0.871 Non-CNS Embolism 0.16 0.21 0.74 (0.42, 1.32 0.308 Myocardial Infarction 1.02 1.11 0.91 (0.72, 1.16) 0.464 All Cause Mortality Vascular Non-vascular Unknown Cause 4.52 2.91 1.15 0.46 4.91 3.11 1.22 0.57 0.92 (0.82, 1.03) 0.94 (0.81, 1.08) 0.94 (0.75, 1.18) 0.80 (0.57, 1.12) 0.152 0.350 0.611 0.195 Vascular Death, Stroke, Embolism Event Rates are per 100 patient-years Based on Intention-to-Treat Population Time in Therapeutic Range (TTR) INR Data Warfarin INR range Median (25th, 75th) <1.5 2.7 (0.0 – 9.0) 1.5 to <1.8 7.9 (3.5 – 14.0) 1.8 to <2.0 9.1 (5.3 – 13.6) 2.0 to 3.0 57.8 (43.0 – 70.5) >3.0 to 3.2 4.0 (1.9 – 6.5) >3.2 to 5.0 7.9 (3.3 – 13.8) >5.0 0.0 (0.0 – 0.5) Based on Rosendaal method with all INR values included Based on Safety Population Primary Efficacy Outcome by Quartiles of cTTR Stroke and non-CNS Embolism Rivaroxaban Warfarin Center TTR Events % Event Rate Events % Event Rate HR (95% CI) 0.0 - 50.6% 2.6 1.8 3.7 2.5 0.71 (0.48, 1.03) 50.7 - 58.5% 3.0 1.9 3.5 2.2 0.83 (0.62, 1.29) 58.6 - 65.7% 3.1 1.9 3.5 2.1 0.92 (0.62, 1.28) 65.7 - 100.0% 2.2 1.3 3.0 1.8 0.77 (0.49, 1.12) Based on Rosendaal method with all INR values included Based on Safety Population Event Rates are per 100 patient-years Primary Safety Outcomes Rivaroxaban Warfarin Event Rate Event Rate HR (95% CI) Pvalue 14.91 14.52 1.03 (0.96, 1.11) 0.442 Major 3.60 3.45 1.04 (0.90, 1.20) 0.576 Non-major Clinically Relevant 11.80 11.37 1.04 (0.96, 1.13) 0.345 Major and non-major Clinically Relevant Event Rates are per 100 patient-years Based on Safety on Treatment Population Primary Safety Outcomes Rivaroxaban Warfarin Event Rate or N (Rate) Event Rate or N (Rate) HR (95% CI) Pvalue 3.60 2.77 1.65 0.82 0.24 3.45 2.26 1.32 1.18 0.48 1.04 (0.90, 1.20) 1.22 (1.03, 1.44) 1.25 (1.01, 1.55) 0.69 (0.53, 0.91) 0.50 (0.31, 0.79) 0.576 0.019 0.044 0.007 0.003 55 (0.49) 84 (0.74) 0.67 (0.47, 0.94) 0.019 Intraparenchymal 37 (0.33) 56 (0.49) 0.67 (0.44, 1.02) 0.060 Intraventricular 2 (0.02) 4 (0.04) Subdural 14 (0.13) 27 (0.27) 0.53 (0.28, 1.00) 0.051 Subarachnoid 4 (0.04) 1 (0.01) Major >2 g/dL Hgb drop Transfusion (> 2 units) Critical organ bleeding Bleeding causing death Intracranial Hemorrhage Event Rates are per 100 patient-years Based on Safety on Treatment Population Adverse Events and Liver Enzyme Data Rivaroxaban (N=7111) Warfarin (N=7125) Any Adverse Event Any Serious Adverse Event AE leading to study drug discontinuation 82.4 37.3 15.7 82.2 38.2 15.2 Epistaxis Peripheral edema Dizziness Nasopharyngitis Cardiac failure Bronchitis Dyspnea Diarrhea 10.1 6.1 6.1 5.9 5.6 5.6 5.3 5.3 8.6 6.2 6.3 6.4 5.9 5.9 5.5 5.6 ALT Elevation >3 x ULN >5 x ULN >3 x ULN and T Bili > 2 x ULN 2.9 1.0 0.4 2.9 1.0 0.5 Values are N (%) Based on Safety Population Summary Efficacy: Rivaroxaban was non-inferior to warfarin for prevention of stroke and non-CNS embolism. Rivaroxaban was superior to warfarin while patients were taking study drug. By intention-to-treat, rivaroxaban was non-inferior to warfarin but did not achieve superiority. Safety: Similar rates of bleeding and adverse events. Less ICH and fatal bleeding with rivaroxaban. Conclusion: Rivaroxaban is a proven alternative to warfarin for moderate or high risk patients with AF. Study Organization IDMC Sponsors J & J and Bayer Christopher Nessel, Kimberly Schwabe, Scott Berkowitz, John Paolini Executive Steering Committee Steering Committee Diego Ardissino, Alvaro Avezum, Phil Aylward, Barbara Biedermann, Christoph Bode, Antonio Carolei, Ramon Corbalan, Laszlo Csiba, Anthony Dalby, Rafael Diaz, Hans Diener, Geoffrey Donnan, Shaun Goodman, Bas Hamer, Hein Heidbuchel, Dai-Yi Hu, Kurt Huber, Gorm Jensen, Matyas Keltai, Basil Lewis, Jose Lopez-Sandon, Jean Louis Mas, Ayrton Massaro, Gordon MacInnes, Bo Norrving, Martin Penicka, Dorairaj Prabhakaran, Risto Roine, Tan Ru San, Per Anton Sirnes, Veronika Skvortsova, Gabriel Steg, Harvey White, Lawrence Wong Duke Clinical Research Institute Jonathan Piccini, Karen Hannan, Jyotsna Garg, Lisa Eskenazi, Angela Kaiser, Patricia Stone Canadian Heart Research Center Shaun Goodman Maggie Godin-Edgecomb Joe Alpert, Chair Allen Skene, Co-chair Gudrun Boysen John Eikelboom Peter Rothwell CEC Manesh Patel Joni O'Briant Lauren Price