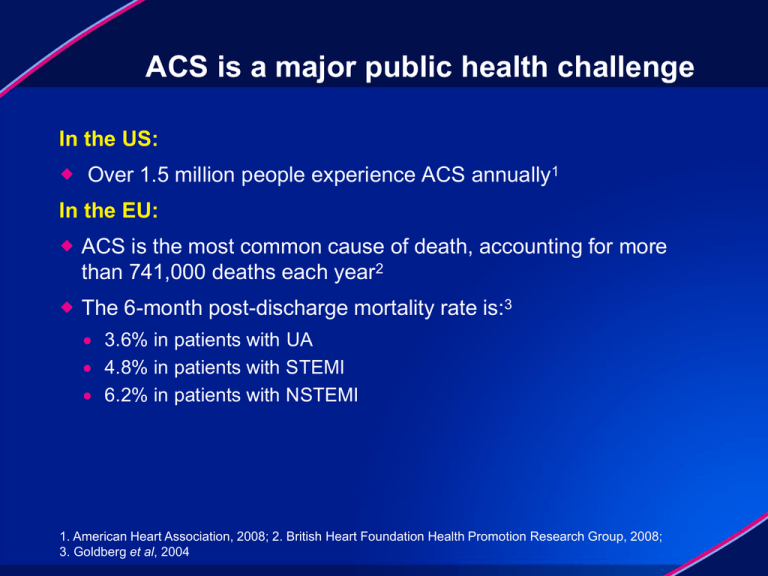
ACS is a major public health challenge
In the US:
Over 1.5 million people experience ACS annually1
In the EU:
ACS is the most common cause of death, accounting for more
than 741,000 deaths each year2
The 6-month post-discharge mortality rate is:3
3.6% in patients with UA
4.8% in patients with STEMI
6.2% in patients with NSTEMI
1. American Heart Association, 2008; 2. British Heart Foundation Health Promotion Research Group, 2008;
3. Goldberg et al, 2004
Event rate of CV death, MI or stroke at
12 months post event remains ~10%
25
CV death, MI or stroke
Major bleeding
Event rate (%)
20
–25%
15
–20%
–16%
–19%
10
5
0
20.0
0.8
None
1.3
15.0
ASA1,2
12.1
1.8*
9.9
2.4*
11.7
2.2*
9.8
2.8*
ASA +
ASA +
ASA +
ASA +
3
3
4
clopidogrel prasugrel clopidogrel ticagrelor4
*Major bleeding: non-CABG-related TIMI major bleeding
1. Antiplatelet Trialists' Collaboration, 1994; 2. Antithrombotic Trialists' Collaboration, 2002; 3. Wiviott et al, 2007;
4. Wallentin et al, 2009
Anticoagulants and antiplatelets have different
yet complementary mechanisms of action
Anticoagulants
Rivaroxaban
Apixaban
Edoxaban
Coagulation
cascade
Platelets
Collagen +
other mediators
Factor
Xa
Thromboxane
Thrombin
ASA
Clopidogrel
Prasugrel
Ticagrelor
ADP
Inflammation
Cellular
proliferation
Antiplatelets
Thrombin
Activated
platelet
Fibrinogen
Platelet
aggregation
Fibrin
Clot
Mackman, 2008
GPIIb/IIIa
inhibitors
GPIIb/IIIa
Rivaroxaban has the potential to further
improve secondary prevention of ACS
Oral administration
O
O
N
N
O
O
Predictable pharmacology
Cl
S
H
N
O
Rivaroxaban
Rapid onset of action
Fixed dose
Balanced dual mode of
elimination
Low potential for drug–drug
or food–drug interactions*
No routine coagulation
monitoring
Rivaroxaban binds directly to the active
site of Factor Xa (Ki 0.4 nM)
*For
full details, see the rivaroxaban Summary of Product Characteristics, available at
http://www.xarelto.com
Roehrig et al, 2005; Perzborn et al, 2005; Kubitza et al, 2005; 2006a,b,c; 2007a,b
2.5 and 5 mg bid rivaroxaban doses were chosen for
phase III based on data from ATLAS ACS TIMI 46
Rivaroxaban 2.5 and 5 mg TIMI major bleeding
Rivaroxaban 2.5 and 5 mg death, MI, stroke
Placebo TIMI major bleeding
Placebo death, MI, stroke
15
5
ASA
11.9%
Incidence (%)
12
9
6
ASA plus thienopyridine
4
3.8%
HR=0.54
(0.27–1.08) 3
HR=0.55
(0.27–1.11)
6.6%
2.0%
2
p=0.17
1.2%
1
3
p=0.03
0
0
90
1.2%
0.0%
180
0.2%
0
0
Time after start of treatment (days)
Gibson, 2008; Data on file at Johnson & Johnson; Mega et al, 2009
90
180
ATLAS ACS 2 TIMI 51
N=15,526*
Physician's decision to add thienopyridine or not
Stratum 1: ASA
alone (7%)
Placebo
n=355
Rivaroxaban
2.5 mg bid
n=349
ASA dose =
75–100 mg/day
Rivaroxaban
5 mg bid
n=349
Stratum 2: ASA +
thienopyridine (93%)
Placebo
n=4821
Rivaroxaban
2.5 mg bid
n=4825
Rivaroxaban
5 mg bid
n=4827
Event-driven study – 1002 events
*184 patients were excluded from the efficacy analyses prior to unblinding because of trial misconduct
at three sites
Mega et al, 2011
Main inclusion and exclusion criteria
Inclusion criteria
Diagnosis of STEMI, NSTEMI, or
UA with at least one of the
following:
≥0.1 mV ST-segment deviation
TIMI risk score ≥4
Patients aged ≥18 years;
<55 years only with either:
Diabetes mellitus or
Prior MI
Patients received ASA
75–100 mg/day alone or ASA
plus a thienopyridine
Based on national/local
dosing guidelines
Gibson et al, 2011
Exclusion criteria
Increased bleeding risk, e.g.
Low platelet count
History of intracranial
haemorrhage
Active internal bleeding
Prior stroke or TIA in stratum 2
patients
Atrial fibrillation: except single
episodes >2 years previously in
patients aged <60 years with no
evidence of cardiopulmonary
disease
Study endpoints/analyses
Primary efficacy endpoint: composite of cardiovascular death,
MI and stroke (ischaemic, haemorrhagic or uncertain)
Main safety endpoint: incidence of major bleeding not
associated with CABG surgery (according to TIMI bleeding
definition)
Primary analysis: log-rank test stratified by thienopyridine use in
mITT population with confirmation in an ITT analysis
mITT: all randomized patients and events from randomization up to
earliest date of completion of treatment period (i.e. global treatment
end date), 30 days after early discontinuation of study drug, or 30 days
after randomization (patients randomized but not treated)
ITT: all randomized patients and events observed from randomization
up to global treatment end date
Gibson et al, 2011
Patient characteristics
Rivaroxaban
2.5 mg bid
(n=5174)
Rivaroxaban
5 mg bid
(n=5176)
Placebo
(n=5176)
62 (9)
62 (9)
62 (9)
Male sex, %
75
74
75
Median weight, kg
78
78
78
Median CrCl, ml/min
85
85
86
Prior MI
26
27
27
Hypertension
67
68
68
Diabetes mellitus
32
32
32
STEMI
50
50
51
NSTEMI
26
26
26
UA
24
24
24
PCI or CABG for index
61
60
60
Mean age, years (SD)
Medical history, %
Index diagnosis, %
Mega et al, 2011
Primary efficacy endpoint (CV death/MI/stroke)
Both rivaroxaban doses, both strata
Estimated cumulative rate (%)
12
2-year Kaplan–Meier estimate
10.7%
10
Placebo
8.9%
8
Rivaroxaban
6
HR=0.84
(0.74–0.96)
ARR=1.7%
mITT p=0.008
4
ITT p=0.002
NNT=56
2
0
0
Number at risk
Placebo
5113
Rivaroxaban 10,229
Mega et al, 2011
4
4307
8502
16
8
12
Months after randomization
3470
6753
2664
5137
1831
3554
20
24
1079
2084
421
831
Primary efficacy analysis: patient subgroups
Both rivaroxaban doses, both strata
HR (95% CI)
Pinteraction
Overall
0.84 (0.74 0.96)
ASA
ASA + thienopyridine
0.69 (0.45 -1.05)
0.86 (0.75 -0.98)
0.34
<65 years
≥65 years
0.83 (0.70 - 0.99)
0.94
STEMI
NSTEMI
UA
0.85 (0.70 - 1.03)
0.85 (0.68 - 1.06)
0.82 (0.62 - 1.07)
0.96
Male
Female
0.87 (0.75 - 1.01)
0.40
Weight <60 kg
Weight 60 to <90 kg
Weight ≥90 kg
0.83 (0.56 - 1.25)
Prior MI
No prior MI
0.83 (0.68 - 1.01)
Diabetes mellitus
No diabetes mellitus
0.96 (0.77 - 1.20)
CrCl <50 ml/min
CrCl ≥50 ml/min
0.88 (0.62 - 1.26)
North America
South America
Western Europe
Eastern Europe
Asia
Other
0.57 (0.33 - 0.97)
Mega et al, 2011
0.84 (0.70 - 1.01)
0.77 (0.60 - 0.99)
0.98
0.85 (0.72 - 0.99)
0.83 (0.64 - 1.08)
0.80
0.85 (0.72 - 1.01)
0.14
0.78 (0.67 - 0.92)
0.82
0.84 (0.73 - 0.96)
0.89 (0.59 - 1.34)
0.90 (0.59 - 1.37)
0.83 (0.69 - 1.00)
0.86 (0.63 - 1.17)
0.92 (0.60 - 1.39)
0.5
0.8
Favours rivaroxaban
1.0
1.25
2.0
Favours placebo
0.80
Primary efficacy endpoint
Separate rivaroxaban doses, both strata
Rivaroxaban
2.5 mg bid
(n=5114)
Rivaroxaban
5 mg bid
(n=5115)
Placebo
(n=5113)
9.1%
8.8%
10.7%
0.84 (0.72–0.97)
0.85 (0.73–0.98)
0.02
0.03
2.7%
4.0%
0.66 (0.51–0.86)
0.94 (0.75–1.20)
0.002
0.63
6.1%
4.9%
0.90 (0.75–1.09)
0.79 (0.65–0.97)
0.27
0.02
1.4%
1.7%
1.13 (0.74–1.73)
1.34 (0.90–2.02)
0.56
0.15
Composite primary endpoint
K–M estimate at 2 years
HR versus placebo (95% CI)
p value versus placebo
CV death
K–M estimate at 2 years
HR versus placebo (95% CI)
p value versus placebo
4.1%
MI
K–M estimate at 2 years
HR versus placebo (95% CI)
p value versus placebo
6.6%
Stroke (haemorrhagic and ischaemic)
K–M estimate at 2 years
HR versus placebo (95% CI)
p value versus placebo
Mega et al, 2011
1.2%
Components of primary endpoint
Rivaroxaban 2.5 mg bid, both strata
CV death/MI/stroke
13
Cardiovascular death
5
HR=0.84
Cumulative incidence (%)
mITT p=0.02
ITT p=0.007
Placebo
All-cause death
5
HR=0.66
mITT p=0.002
ITT p=0.005
10.7%
HR=0.68
Placebo
mITT p=0.002
ITT p=0.004
4.1%
Placebo
4.5%
9.1%
2.9%
2.7%
Rivaroxaban
2.5 mg bid
Rivaroxaban
Rivaroxaban
2.5 mg bid
2.5 mg bid
NNT=63
0
NNT=71
0
0
6
12
18
Months
24
Mega et al, 2011; Gibson et al, 2011
NNT=63
0
0
6
12
18
Months
24
0
6
12
18
Months
24
Stent thrombosis*
Both rivaroxaban, both strata
2-year Kaplan–Meier estimate
3
2.9%
Estimated cumulative incidence (%)
Placebo
2.3%
2
Rivaroxaban
HR=0.69
(0.51–0.93)
RRR=31%
mITT p=0.02
ITT p=0.008
1
2-year K–M estimate
HR versus placebo (95% CI)
p value vs placebo (mITT)
Rivaroxaban
2.5 mg bid
Rivaroxaban
5 mg bid
2.2%
2.3%
0.65 (0.45–0.94) 0.73 (0.51–1.04)
0.02
0.08
0
0
4
8
12
16
Months after randomization
20
24
*Stent thrombosis events: definite, probable or possible (Academic Research Consortium definitions)
Mega et al, 2011
Principal safety endpoint
Separate rivaroxaban doses, both strata
Non-CABG TIMI major bleed
K–M estimate at 2 years
p value versus placebo
ICH
K–M estimate at 2 years
p value versus placebo
Fatal bleeding
K–M estimate at 2 years
p value versus placebo
Fatal ICH
K–M estimate at 2 years
p value versus placebo
Mega et al, 2011
Rivaroxaban
2.5 mg bid
(n=5115)
Rivaroxaban
5 mg bid
(n=5110)
1.8%
<0.001
2.4%
<0.001
0.6%
0.4%
0.04
0.7%
0.005
0.2%
0.1%
0.45
0.4%
0.20
0.2%
0.1%
–
0.2%
–
0.1%
Placebo
(n=5125)
Treatment-emergent fatal bleeding events and ICH
2-year Kaplan–Meier estimate (%)
Separate rivaroxaban doses, both strata
Placebo
2.5 mg rivaroxaban
5.0 mg rivaroxaban
1.0 Rivaroxaban vs placebo Rivaroxaban vs placebo Rivaroxaban vs placebo
p=NS
p=0.009
p=NS
0.8
0.7
0.6
0.4
0.4
0.2
0.2
0.4
0.2
0.2
0.1
0.1
0.1
0.0
Fatal bleeding events
Mega et al, 2011
ICH
Fatal ICH
Other safety endpoints
Separate rivaroxaban doses, both strata
Rivaroxaban
2.5 mg bid
(n=5115)
Rivaroxaban
5 mg bid
(n=5110)
Placebo
(n=5125)
Post-treatment ischaemic events*
Raw percentage
1.4%
2.2%
p value versus placebo
p=NS
p=NS
1.8%
Liver function test (ALT >3× ULN)#
Raw percentage
1.3%
1.4%
p value versus placebo
p=NS
p=NS
1.6%
Other adverse events (raw percentages)
Dyspnoea
1.1%
1.3%
1.5%
Cough
1.2%
1.1%
1.4%
*CV death/MI/stroke (ischaemic, haemorrhagic, uncertain) events occurring 1–10 days after last
rivaroxaban dose; #Abnormal values from first dose to 2 days post last dose in patients
with normal baseline values
Mega et al, 2011
ATLAS ACS 2 TIMI 51: summary
Compared with placebo, rivaroxaban (2.5 or 5 mg bid) on top of
ASA or ASA plus clopidogrel showed:
Significant reductions in the rates of death, MI , and stroke
Benefits in all types of ACS patients (UA, NSTEMI and STEMI )
More than a 30% reduction in risk of both CV and all-cause mortality
(2.5 mg bid)
No increase in fatal bleeding and fatal ICH
A non-bleeding safety profile similar to placebo
The addition of anticoagulation with rivaroxaban may represent a
new treatment strategy in patients after recent ACS
Mega et al, 2011