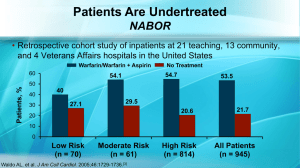
Anticoagulation
advertisement

Br J Haematol. 2008 Jun;141(6):757-63 A review of guidelines and update in emerging therapies Brian Spoelhof, PharmD April 19, 2012 The presenter has no actual or potential conflicts to disclose Summarize the indications for anticoagulation Describe the pharmacology of new oral anticoagulants Evaluate the data that led to the approval of the new oral anticoagulants Discuss the advantages and disadvantages of new anticoagulants; Examine new potential indications for the new anticoagulants. Anticoagulation Guidelines Atrial Fibrillation Post-op Orthopedic Surgery Pharmacology of current options Dabigatran Rivaroxaban Apixiban Summary Questions Safe Effective Oral Easy Reversible Tissue Damage Surface Contact Common Pathway Dipiro: Pharmacotherapy: a Pathophysiologic Approach, 2008 Vitamin K Antagonist Unfractionated Heparin (UFH) Low Molecular Weight Heparin (LMWH) Direct Thrombin Inhibitors Factor Xa Inhibitors Warfarin Heparin Enoxaparin Bivalirudin Argatroban Dabigatran Fondaparinux Rivaroxaban Apixiban Vitamin K Antagonist Narrow Therapeutic Genetic variation Drug interactions Food interactions Required monitoring Slow onset of action Protein Half Life (Hours) Prothrombin (II) 60-100 Factor VII 6-8 Factor IX 20-30 Factor X 24-40 Protein C 8-10 Protein S 40-60 Is this the perfect anticoagulant? Dipiro: Pharmacotherapy: a Pathophysiologic Approach, 2008 AAOS – American Academy of Orthopedic Surgeons Updated September 2011 Recommends no specific agent ACCP – American College of Chest Physicians Updated February 2012 Hip Fracture Surgery Total Hip Replacement Total Knee Replacement Chest. 2008 Jun;133 AAOS VTE Prevention Guidelines LMWH (preferred), Fondaparinux, Warfarin (INR 2-3), Dabigatran*, Rivaroxaban*, Apixaban* * Not recommend in hip fracture surgery ACCP and ACCF/AHA /HRS guidelines fairly similar Risk Stratification C – Congestive heart failure H - Hypertension A – Age ≥ 75 D - Diabetes Sx2 – Prior stroke or TIA x 2 J Am Coll Cardiol. 2011 Mar 15;57(11):1330-7 Chest. 2008 Jun;133 CHADS2 score of 0 CHADS2 score of 1 Aspirin 81 to 325 mg daily Aspirin 81 to 325 mg daily plus clopidogrel or Dabigatran or warfarin titrated to INR of 2.0-3.0 CHADS2 score 2 or greater Dabigatran or warfarin titrated to INR of 2.0-3.0 J Am Coll Cardiol. 2011 Mar 15;57(11):1330-7 Chest. 2008 Jun;133 Oral anticoagulation preferred over dual antiplatelet therapy Dabigatran preferred over warfarin, except Mitral valve stenosis Stable coronary artery disease Intracoronary stents Dabigatran – Pradaxa Rivaroxaban – Xarelto Direct Thrombin Inhibitor Approved to prevent stroke and systemic embolism nonvalvular atrial fibrillation Factor Xa Inhibitor Approved to prevent stroke and systemic embolism nonvalvular atrial fibrillation and Postoperative thromboprophylaxis Apixaban Factor Xa Inhibitor Not currently approved Rivaroxaban, Package Insert Dabigatran, Package Insert Indication: Dosage: Prevent stroke and systemic embolism nonvalvular atrial fibrillation CrCl > 30 mL/min: 150 mg Twice Daily Renal: Next slide Dyspepsia Dabigatran, Package Insert CrCl 15 – 30 mL/min: 75 mg Twice Daily November 2011 Consider reduced dose (75 md twice daily) in patients with moderate renal impairment (30-50 mL/min) and concurrently taking ketoconazole or dronedarone. Assess renal function prior to starting and in patients ≥ 75 years old CrCl or < 50 mL/min Use with extreme caution in patient greater than 80 Dabigatran, Package Insert Dabigatran Dipiro: Pharmacotherapy: a Pathophysiologic Approach, 2008 Pharmacokinetics Prodrug Rapid absorption Time to peak: 1-2 hours Half-Life: 12-17 hours Longer in renal impairment Dabigatran, Package Insert Monitoring: aPTT Qualitative not Quantitative TT (Thrombin Time) Linear dose relationship Not as readily available Randomized, Dose blinded/regimen unblinded, noninferiority trial Dabigatran 110 mg twice daily vs. Dabigatran 150 mg twice daily vs . Warfarin titrated to INR n = 18,113 N Engl J Med. 2009 Sep 17;361(12):1139-51 N Engl J Med. 2009 Sep 17;361(12):1139-51 N Engl J Med. 2009 Sep 17;361(12):1139-51 Increased efficacy noninferior bleeding Noninferior efficacy lower bleeding No known reversal agent Study of 12 healthy individuals Prothrombin Complex Concentrate No effect on aPTT or TT Supportive care Blood Fluid (to support kidney function) Possible dialysis Circulation. 2011 Oct 4;124(14):1573-9 Oral direct thrombin inhibitor Requires renal adjustments More effective than warfarin Same risk of bleeding Twice daily dosing Dyspepsia Limited available monitoring No reversal Indications: Approved to prevent stroke and systemic embolism nonvalvular atrial fibrillation Postoperative thromboprophylaxis (Knee and Hip) Dosage Afib: CrCl >50 mL/min: 20 mg once daily CrCl 15 - 50 mL/min: 15 mg once daily Post-op VTE prophylaxis Knee replacement: 10 mg once daily x 12-14 days Hip replacement: 10mg once daily x 35 days Rivaroxaban Package Insert Pharmacodynamics Peak 2.5-4 hours Monitoring PT Half Life: 3.2 – 22 hours Metabolized via 3A4 aPTT Anti-Xa Br J Clin Pharmacol. 2011 Oct;72(4):593-603 Thromb Haemost. 2010 Apr;103(4):815-25 More sensitive Varies with different reagents Cannot be standardized Modified Anti-Xa being developed Rivaroxaban directly inhibits Factor Xa Rivaroxaban Dipiro: Pharmacotherapy: a Pathophysiologic Approach, 2008 J Thromb Haemost. 2006 Jan;4(1):121-8 Trial Setting Enoxaparin regimen Rivaroxaban DVT/PE/ regimen death (%) RRR Symptomatic RRR (%) VTE (%) (%) RECORD1 THA n=4541 40 mg daily x 10 mg daily x 3.7 vs 1.1 35 days 35 days 70 — — RECORD2 THA n=2509 40 mg daily x 10 mg daily x 9.3 vs 2.0 10–14 days 31–39 days 79 1.2 vs 0.2 80 RECORD3 TKA n=2531 40 mg daily x 10 mg daily x 18.9 vs 9.6 10–14 days 10–14 days 49 2.0 vs 0.7 66 RECORD4 TKA n=3148 30 mg BID x 10 mg daily x 10.1 vs 6.9 10–14 days 10–14 days 31 1.2 vs 0.7 NS Eikelboom JS and Weitz JI. Lancet 2008. Outcome Enoxaparin Rivaroxaban p (%) (%) Symptomatic VTE/all-cause mortality Major bleed 1.3 0.5 <0.001 0.2 0.3 0.305 Turpie AG et al. 2008 International Congress on Thrombosis; June 27, 2008; Athens, Greece. Abstract O5. Comparison of rivaroxaban to warfarin in patients with atrial fibrillation Randomized, Double Blinded, Double Dummy, Noninferiority Consideration Time in Therapeutic Range N Engl J Med. 2011 Sep 8;365(10):883-91 N Engl J Med. 2011 Sep 8;365(10):883-91 N Engl J Med. 2011 Sep 8;365(10):883-91 Prothrombin Complex Concentrate potentially reverses rivaroxaban Study in 12 healthy males Returned to nearly normally levels within 15 minutes Circulation. 2011 Oct 4;124(14):1573-9 Oral direct Factor Xa inhibitor Post-op thromboprophylaxis Superior to enoxaparin Similar rates of major bleeds Stroke prophylaxis in atrial fibrillation Non-inferior to warfarin Less risk of major bleeding Discontinuation increases risk of thromboembolism Anticoagulation rapidly evolving New option provide potential but haven’t eradicated the need for warfarin When choosing an agent must balance compliance, risk, renal function Oral Factor Xa inhibitor Not yet approved, no indications Approval expected 6/28/12 Dosing: 5 mg twice daily 2.5 mg twice daily with two of the following: Age > 80 years Weight < 60 kg SCr > 1.5 mg/dL Apixaban vs warfarin for atrial fibrillation Randomized, double blind, double dummy, noninferiority trial n= 18,201patient Apixaban awaiting FDA review Approval expected Apixaban reduced occurrence of stroke and systemic embolism compared to warfarin Apixaban associated with lower risk of bleeding compared to warfarin Warfarin has reduced secondary endpoints but risk of bleeding has not outweighed benefit APPRAISE-2 Apixaban 5 mg BID vs Placebo post- MI No benefit ATLAS-ACS2 TIMI 51 Rivaroxaban 2.5 mg daily or 5 mg daily vs placebo postMI Rivaroxaban 2.5 mg = Benefit Rivaroxaban 5 mg = No benefit Hurlen M, et al. N Engl J Med. 2002 Sep 26;347(13):969-74 Rivaroxaban 2.5 mg daily Decreased primary endpoint Cardiovascular Death, MI, or stroke 9.1% vs 10.7% (HR 0.84, P=0.0.02) NNT = 63 Decreased all cause mortality 2.9 % vs 4.5 % (HR 0.68, P=0.002) NNT = 63 Increased major bleeding (HR 3.46, P=0.001) 1.8% vs 0.6%(HR 3.46, P=0.001) NNH = 83 Dabigatran • Best stroke reduction data • Twice daily dosing • Dyspepsia/ GI Bleed • No reversal Rivaroxaban • Reversible • Once daily dosing • Afib data not as strong • Early discontinuation increases events Apixaban • Better efficacy and safety • Theoretically reversible • Twice daily dosing • Not yet approved DiPiro, Joseph T. Pharmacotherapy: a Pathophysiologic Approach. New York: McGraw-Hill Medical, 2008. Print. Katzung, Bertram G. Basic and Clinical Pharmacology. New York: McGraw Hill Medical, 2007. Print. Jacobs, J; Mont, M; Bozic, K; et al, Guideline on Preventing Venous Thromboembolic Disease in Patients Undergoing Elective Hip and Knee Arthroplasty. Rosemont, IL AAOS September 24, 2011 http://www.jointcommission.org/specifications_manual_for_national_hospital_inpatient_quality_mea sures/ - Specifications Manual for National Hospital Inpatient Quality Measure; The Joint Commission Surgical Care Improvement Dabigatran [package insert]. Boehringer Ingelheim Pharmaceuticals, Inc. Ridgefield, CT, November, 2011 http://bidocs.boehringeringelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Informati on/PIs/Pradaxa/Pradaxa.pdf Rivaroxaban [package insert Janssen Pharmaceuticals, Inc. Titusville, NJ 2011 http://www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf Eriksson BI, Quinlan DJ, Eikelboom JW. Novel oral factor Xa and thrombin inhibitors in the management of thromboembolism. Annu Rev Med. 2011 Feb 18;62:41-57. Turpie AG, Lassen MR, Kakkar AK, et al. A meta-analysis of three pivotal studies of rivaroxaban—a novel, oral, direct factor XA inhibitor—for thromboprophylaxis after orthopaedic surgery. 2008 International Congress on Thrombosis; June 27, 2008; Athens, Greece. Abstract O56. Eikelboom JW and Weitz JI. Selective factor Xa inhibition for thromboprophylaxis. Lancet 2008; DOI:10.1016/S0140-6736(08)60879-X. Available at: http://www.thelancet.com. Eriksson BI, Borris L, Dahl OE, Haas S, Huisman MV, Kakkar AK, Misselwitz F, Kälebo P; ODIXa-HIP Study Investigators. Oral, direct Factor Xa inhibition with BAY 59-7939 for the prevention of venous thromboembolism after total hip replacement. J Thromb Haemost. 2006 Jan;4(1):121-8. PubMed PMID: 16409461. Wardrop D, Keeling D. The story of the discovery of heparin and warfarin. Br J Haematol. 2008 Jun;141(6):757-63. Epub 2008 Mar 18. Review. PubMed PMID: 18355382. Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ, American College of Chest Physician.Antithrombotic and thrombolytic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008 Jun;133(6 Suppl):110S-112S. Erratum in: Chest. 2008 Aug;134(2):473. PubMed PMID: 18574260. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Sep 17;361(12):1139-51. Epub 2009 Aug 30. Erratum in: N Engl J Med. 2010 Nov 4;363(19):1877. PubMed PMID: 19717844. Barry M. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009 Dec 31;361(27):2674; author reply 2675. PubMed PMID: 20050385. Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, Perzborn E. Assessment of laboratory assays to measure rivaroxaban--an oral, direct factor Xa inhibitor. Thromb Haemost. 2010 Apr;103(4):815-25. Epub 2010 Feb 2. PubMed PMID: 20135059. Wann LS, Curtis AB, Ellenbogen KA, Estes NA 3rd, Ezekowitz MD, Jackman WM, January CT, Lowe JE, Page RL, Slotwiner DJ, Stevenson WG, Tracy CM. 2011 ACCF/AHA/HRS focused update on the management of patients with atrial fibrillation (update on dabigatran): a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2011 Mar 15;57(11):1330-7. Epub 2011 Feb 14. PubMed PMID: 21324629. Kazmi RS, Lwaleed BA. New anticoagulants: how to deal with treatment failure and bleeding complications. Br J Clin Pharmacol. 2011 Oct;72(4):593-603. doi: 10.1111/j.1365-2125.2011.04060.x. PubMed PMID: 21752066; PubMed Central PMCID: PMC3195736. Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011 Oct 4;124(14):1573-9. Epub 2011 Sep 6. PubMed PMID: 21900088. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011 Sep 8;365(10):883-91. Epub 2011 Aug 10. PubMed PMID: 21830957. Hurlen M, Abdelnoor M, Smith P, Erikssen J, Arnesen H. Warfarin, aspirin, or both after myocardial infarction. N Engl J Med. 2002 Sep 26;347(13):969-74