Molecular Formula - Prairie Spirit Blogs
advertisement

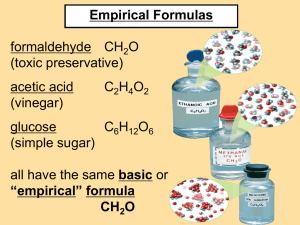
4.6 MOLECULAR FORMULAS 2 3 Steps for determining Chemical Formulas 1. Determine the percent composition of all elements. 2. Convert this information into an empirical formula 3. Find the true number of atoms/ elements in the compound (Molecular Formula) 4.6 Molecular Formula • Molecular Formula of a compound tells you exact number of atoms in one molecule of a compound. This formula may be equal to the empirical formula or may be a multiple of this formula. • To determine, you need: • The empirical formula • The molar mass of the compound • Empirical Formula - shows the ratio between atoms Example: CH2O • Molecular Formula - shows the actual number of atoms Example: C6H12O6 Steps to Determine Molecular Formula 1. List the given values (if the empirical formula is not given, you must determine that before moving on). 2. Determine the molar mass for the empirical formula. 3. Divide the Molecular molar mass by the empirical formula molar mass. 4. Calculate Molecular Formula by multiplying this number by the empirical formula. Example 1: The empirical formula of a compound is CH3O and its molar mass is 93.12g/mol. What is the molecular formula? Step 1: List given values Empirical Formula=CH3O Mcompound = 93.12 g/mol Step 2: Determine the molar mass for the empirical formula, CH3O. MEmpirical = 12.01g/mol + 3(1.01g/mol) + 16.00g/mol = 31.04 g/mol Step 3. Divide the Molecular molar mass by the empirical formula molar mass. Molecular formula molar mass Empirical formula molar mass = 93.12 g/mol 31.04 g/mol Step 4. Calculate Molecular Formula by multiplying this number by the empirical formula. Molecular formula = x (empirical formula) 3 x CH3O Therefore, the molecular formula is C3H9O3 Example 2: The percent composition of a compound is determined by a combustion and analyzer is a 40.03% carbon, 6.67% hydrogen, & 53.30% oxygen. The molar mass is 180.18g/mol. What is the molecular formula Step 1: List given values C= 40.03%, O=53.30%, H=6.67% Mcompound = 180.18 g/mol The empirical formula is not given so you must calculate it now. a. Calculate the mass of each element in a 100g sample mC=40.03g mO=53.30g mH=6.67g b. Convert Mass (m) into moles (n) nC= 40.03g x 1mol/ 12.01g = 3.33 mol C nH= 6.67g x 1 mol/ 1.01g = 6.60 mol H nO= 53.30g x 1 mol/ 16.00g = 3.33 mol O c. State the Amount Ratio nC : nH : nO 3.33mol : 6.60mol : 3.33 mol d. Calculate lowest whole number ratio 3.33mol : 6.60mol : 3.33 mol 3.33mol : 3.33mol : 3.33 mol 1 : 2: 1 Empirical Formula is CH2O Step 2: Determine the molar mass for the empirical formula MEmpirical = 12.01g/mol + 2(1.01g/mol) + 16.00g/mol = 30.03 g/mol Step 3. Divide the molar mass by the empirical formula molar mass. Molar mass = 180.18 g/mol Empirical formula molar mass 30.03 g/mol Step 4. Calculate Molecular Formula by multiplying this number by the empirical formula. Molecular formula = x (empirical formula) 6 x (CH2O) Therefore, the molecular formula is C6H12O6 Example 3: The percent composition of a compound is determined by a combustion analyzer is a 32.0% carbon, 6.70% hydrogen, 42.6% oxygen & 18.7% nitrogen. The molar mass is 75.08g/mol. What is the molecular formula? List given values C = 32.0% H = 6.70% Mformula = 75.08g/mol 1. O = 42.6% N = 18.7% *** Empirical Formula is not given so you must now calculate it! Calculate the mass of each element in a 100g sample mC=32.0g mO=42.6g mH=6.70g mN=18.7g Convert Mass (m) into moles (n) nC= 32.0g x 1mol/ 12.01g = 2.66 mol C nH= 6.70g x 1mol/ 1.01g = 6.65 mol H nO= 42.6g x 1mol/ 16.00g = 2.66 mol O nN= 18.7g x 1mol/14.01g = 1.33 mol N State the Amount Ratio nC : nH : nO 2.66mol : 6.65mol : 2.6 mol : nN : 1.33mol Calculate lowest whole number ratio 2.66mol : 6.65mol : 2.6 mol: 1.33mol 1.33mol : 1.33mol : 1.33 mol: 1.33mol 2:5:2:1 Empirical Formula is C2H5O2N 2. Determine the molar mass for the empirical formula MEmpirical = 75.08g 3. Divide the molar mass by the empirical formula molar mass. Molar mass = Empirical formula molar mass 75.08 g/mol 75.08 g/mol 4. Calculate Molecular Formula by multiplying this number by the empirical formula. Molecular formula = x (empirical formula) 1 x (C2H5O2N) Therefore, the molecular formula is C2H5O2N Assignment • Proceed with the assignment that follows Molecular Formula Check Point 1. The empirical formula of a compound is CH2. Its molecular mass is 70g/mol. What is its molecular formula? 2. A compound is found to be 40.0% carbon, 6.7% hydrogen and 53.5% oxygen. Its molecular mass is 60.0g/mol. What is its molecular formula? Molecular Formula Check Point #2 1. A class of compounds called sodium metaphosphates were used as additives to detergents to improve cleaning ability. One of them has a molecular mass of612g. Analysis shows the composition to be 22.5% Na, 30.4% P, and 47.1 % O. Determine the molecular formula of this compound