Buffer Solutions
advertisement

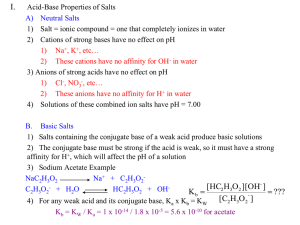
Drill: A 0.100 M solution of HZ ionizes 20.0 %. Calculate: KaHZ Buffer Solutions Buffer Solution •A solution that resists changes in pH Buffer Solution •Made from the combination of a weak acid & its salt Buffer Solution •Made from the combination of a weak base & its salt Buffer Examples •Mix acetic acid & sodium acetate •Mix ammonia & ammonium chloride Buffer Solution •A buffer solution works best when the acid to salt ratio is 1:1 Buffer Solution •A buffer solution works best when the base to salt ratio is 1:1 Buffer Solution •The buffering capacity of a solution works best when the pH is near the pKa pKa or pKb •pKa = - log Ka •pKb = - log Kb Buffer Equilibria To solve buffer equilibrium problems, use the same 5 steps 5 Steps of Equilibrium Problems 1) Set up & balance reaction 5 Steps of Equilibrium Problems 2) Assign Equilibrium amounts in terms of x (ICE) 5 Steps of Equilibrium Problems 3) Write the equilibrium expression (K = ?) 5 Steps of Equilibrium Problems 4) Substitute Equilibrium amounts into the K 5 Steps of Equilibrium Problems 5) Solve for x Buffer Problems •Calculate the pH of a solution containing 0.10 M HAc in 0.10 M -5 NaAc: Ka = 1.8 x 10 Buffer Problems •Calculate the pH of 0.10 M NH3 in 0.20 M NH4NO3: -5 •Kb = 1.8 x 10 Buffer Problems Calculate the pH of a solution containing 0.10 M HBz in 0.20 M -5 NaBz: Ka = 6.4 x 10 Drill: Calculate the pH of a solution containing 0.30 M HZ in 0.10 M -5 NaZ: Ka = 3.0 x 10 Buffer Problem Calculate the pH of a solution containing 0.50 M R-NH2 in 0.10 M -5 R-NH3I: Kb = 4.0 x 10 Derivations from an equilibrium constant HA + H + A [ ][ ] Ka = [HA] + H A HA + H +A [ ][ ] Ka = [HA] + H A Cross multiply to + isolate [H ] HA + H +A [ K ][ HA ] a + [H ]= [A ] HA + H +A [ HA ] + [H ] = (Ka) [A ] HA + H +A [HA] + [H ] = (Ka) [A ] Take –log of each side pH = [HA] pKa - log [A ] HendersonHasselbach Eq [A ] pH = pKa + log [HA] HendersonHasselbach Eq + [B ] pOH = pKb+ log [B] Buffer Problems •Calculate the salt to acid ratio to make a buffer solution with pH = 5.0 -5 •Ka for HBZ = 2.0 x 10 Derivations from an equilibrium constant HA + H +A [ ][ ] Ka = [HA] + H A [ ][ ] Ka = [HA] + H A Divide both sides by + [H ] Ka [ ] = + [H ] [HA] A You Get the Salt to Acid Ratio Drill: •Calculate the salt to acid ratio to make a buffer solution with pH = 5.0 -5 •Ka for HBZ = 2.0 x 10 Buffer Problems Calculate the salt to base ratio to make a buffer solution with pH = 9.48 -5 •Kb for MOH = 2.0 x 10 Equivalence Point Point at which the # of moles of the two titrants are equal Titration Curves 14 12 10 8 6 4 2 0 0.00 10.00 20.00 30.00 40.00 50.00 14 12 10 8 [HA]=[OH-] 6 4 [HA]=[A-] 2 0 0.00 10.00 20.00 30.00 40.00 50.00 Drill: Calculate the pH of a buffer solution containing 0.50 M HX in 0.25 M KX. -5 Ka = 2.5 x 10 14 12 [OH-] = [A-2] 10 8 [HA-] = [A-2] 6 [H2A] = [OH-] 4 2 [H2A] = [HA-] 0 0 20 40 60 80 100 - Calculate the HCO3 to H2CO3 ratio in blood with pH = 7.40 -7 •Ka1 for H2CO3 = 4.4 x 10 150 ml of 0.10 M NaOH is added to 100.0 ml of 0.10 M H2CO3. Calculate pH. -7 •Ka1 for H2CO3 = 4.4 x 10 -11 •Ka2 for H2CO3 = 4.8 x 10