Acid-Base Equilibrium: Monoprotic Acids & Buffers
advertisement

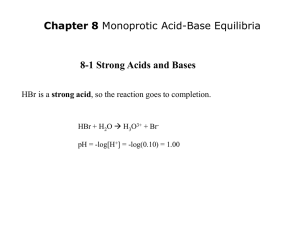
Acid-Base Equilibrium (Monoprotic) Chapter 9 THE TRUTH, THE WHOLE TRUTH AND NOTHING BUT THE TRUTH. Strong Acids and Bases pH + pOH = - log Kw = pKw = 14.00 for Kw = 1.0x10-14 pH + pOH = - log Kw = pKw = 13.996 for Kw = 1.01x10-14 at 25oC EXAMPLE: What is the pH of a 1.0x10-8M solution of HCl? MHCl = 1.0 x 10-8 M [H+]HCl = 1.0 x 10-8 M pH = 8.00 WRONG!!!!!!!! HCl is an acid! Acids have pH less than 7! OH NO! WHAT SHOULD WE DO ! What's wrong? We ignored the fact that water is also an ACID! [H+]total = [H+]water + [H+]HCl It’s BACK ! Systematic Equilibrium near pH 7, the contribution of [H+] from water becomes the dominate source for the [H+] in the solution SO HOW DO WE SOLVE THIS ? [H+]total = [H+]water + [H+]HCl [H+]total = [H+]water + 1.0 x 10-8M let [H+]water = x = [OH-] Kw = [H+]total[OH-] = 1.01 X 10-14 at 25oC [H+]total[OH-] = 1.01 X 10-14 ([H+]water + 1.0 x 10-8 M) [OH-] = 1.01 X 10-14 ( x + 1.0 x 10-8M) x = 1.01 X 10-14 ( x + 1.0 x 10-8M) x = 1.01 X 10-14M2 by quadratic x = 9.51 x 10-8M [H+]total = 9.51 x 10-8M + 1.0 x 10-8M [H+]total = 10.5 x 10-8M = 1.05 x 10-7M pH = 6.98 What is the pH of a 0.5 M HF solution (at 250C)? HF (aq) Initial (M) Change (M) Equilibrium (M) +][F-] [H = 7.1 x 10-4 Ka = H+ (aq) + F- (aq) [HF] HF (aq) H+ (aq) + F- (aq) 0.50 0.00 0.00 -x +x +x 0.50 - x x x x2 = 7.1 x 10-4 Ka = 0.50 - x x2 Ka = 7.1 x 10-4 0.50 [H+] = [F-] = 0.019 M [HF] = 0.50 – x = 0.48 M Ka << 1 0.50 – x 0.50 x2 = 3.55 x 10-4 x = 0.019 M pH = -log [H+] = 1.72 When can I use the approximation? Ka << 1 0.50 – x 0.50 When x is less than 5% of the value from which it is subtracted. x = 0.019 0.019 M x 100% = 3.8% 0.50 M Less than 5% Approximation ok. What is the pH of a 0.05 M HF solution (at 250C)? x2 Ka = 7.1 x 10-4 x = 0.006 M 0.05 More than 5% 0.006 M x 100% = 12% Approximation not ok. 0.05 M Must solve for x exactly using quadratic equation or method of successive approximation. What is the pH of a 0.122 M monoprotic acid whose Ka is 5.7 x 10-4? HA (aq) Initial (M) Change (M) Equilibrium (M) 0.122 0.00 0.00 -x +x +x 0.122 - x x x x2 = 5.7 x 10-4 Ka = 0.122 - x Ka H+ (aq) + A- (aq) x2 = 5.7 x 10-4 0.122 0.0083 M x 100% = 6.8% 0.122 M Ka << 1 0.122 – x 0.122 x2 = 6.95 x 10-5 x = 0.0083 M More than 5% Approximation not ok. x2 = 5.7 x 10-4 Ka = 0.122 - x ax2 + bx + c =0 x = 0.0081 HA (aq) Initial (M) Change (M) Equilibrium (M) x2 + 0.00057x – 6.95 x 10-5 = 0 -b ± b2 – 4ac x= 2a x = - 0.0081 H+ (aq) + A- (aq) 0.122 0.00 0.00 -x +x +x 0.122 - x x x [H+] = x = 0.0081 M pH = -log[H+] = 2.09 Ionized acid concentration at equilibrium percent ionization = x 100% Initial concentration of acid For a monoprotic acid HA Percent ionization = [H+] [HA]0 x 100% [HA]0 = initial concentration Weak Bases and Base Ionization Constants NH3 (aq) + H2O (l) NH4+ (aq) + OH- (aq) [NH4+][OH-] Kb = [NH3] Kb is the base ionization constant Kb weak base strength Solve weak base problems like weak acids except solve for [OH-] instead of [H+]. CHM 112 Summer 2007 Prushan M. Ionization Constants of Conjugate Acid-Base Pairs HA (aq) A- (aq) + H2O (l) H2O (l) H+ (aq) + A- (aq) OH- (aq) + HA (aq) H+ (aq) + OH- (aq) Ka Kb Kw KaKb = Kw Weak Acid and Its Conjugate Base Kw Ka = Kb Kw Kb = Ka 15.7 Fractional Ionization of a Monoprotic Weak Acid Weak acids are those that are not leveled, or completely ionized in the solvent. Acetic acid is a relatively weak acid. The degree of ionization in aqueous solution depends on the formal concentration of HOAc, as well as the existence of other acid or base species that may be in solution. If we divide both sides by volume, and use the definition of formal Which is a mathematical statement of mass balance, i.e., the sum of the molar concentrations of all acetic species equals the formula weights we put into solution. A Little Algebra…. and substituted into the formal mass balance equation to yield The fraction of acid in acetate form is [OAc-]/FHOAc. Solving for this fraction results in the amount of HOAc in the form of the acetate a & a The ratios are often called the "alpha“ (a) of acetate and acetic acid respectively. Fractional Composition (a) plot for acetic acid What is the pH when [HA] = [A-]??? 1 a A( pH ) a HA( pH ) 4.75 0.5 0 0 2 4 6 pH Which is the pKa…. 8 10 12 The common ion effect is the shift in equilibrium caused by the addition of a compound having an ion in common with the dissolved substance. The presence of a common ion suppresses the ionization of a weak acid or a weak base. Consider mixture of CH3COONa (strong electrolyte) and CH3COOH (weak acid). CH3COONa (s) CH3COOH (aq) Na+ (aq) + CH3COO- (aq) H+ (aq) + CH3COO- (aq) common ion Consider mixture of salt NaA and weak acid HA. NaA (s) Na+ (aq) + A- (aq) HA (aq) H+ (aq) + A- (aq) [H+] Ka [HA] = [A-] -log [H+] = -log Ka - log [HA] [A-] -] [A -log [H+] = -log Ka + log [HA] [A-] pH = pKa + log [HA] [H+][A-] Ka = [HA] Henderson-Hasselbalch equation [conjugate base] pH = pKa + log [acid] pKa = -log Ka What is the pH of a solution containing 0.30 M HCOOH and 0.52 M HCOOK? Mixture of weak acid and conjugate base! HCOOH (aq) Initial (M) Change (M) Equilibrium (M) Common ion effect 0.30 – x 0.30 0.52 + x 0.52 HCOOH pKa = 3.77 H+ (aq) + HCOO- (aq) 0.30 0.00 0.52 -x +x +x 0.30 - x x 0.52 + x [HCOO-] pH = pKa + log [HCOOH] [0.52] = 4.01 pH = 3.77 + log [0.30] A buffer solution is a solution of: 1. A weak acid or a weak base and 2. The salt of the weak acid or weak base Both must be present! A buffer solution has the ability to resist changes in pH upon the addition of small amounts of either acid or base. Consider an equal molar mixture of CH3COOH and CH3COONa Add strong acid H+ (aq) + CH3COO- (aq) Add strong base OH- (aq) + CH3COOH (aq) CH3COOH (aq) CH3COO- (aq) + H2O (l) Which of the following are buffer systems? (a) KF/HF (b) KBr/HBr, (c) Na2CO3/NaHCO3 (a) HF is a weak acid and F- is its conjugate base buffer solution (b) HBr is a strong acid not a buffer solution (c) CO32- is a weak base and HCO3- is it conjugate acid buffer solution Calculate the pH of the 0.30 M NH3/0.36 M NH4Cl buffer system. What is the pH after the addition of 20.0 mL of 0.050 M NaOH to 80.0 mL of the buffer solution? NH4+ (aq) [NH3] pH = pKa + log [NH4+] start (moles) end (moles) H+ (aq) + NH3 (aq) pKa = 9.25 0.029 0.001 NH4+ (aq) + OH- (aq) 0.028 0.0 [0.30] pH = 9.25 + log = 9.17 [0.36] 0.024 H2O (l) + NH3 (aq) 0.025 final volume = 80.0 mL + 20.0 mL = 100 mL 0.028 0.025 [0.25] + pH = 9.25 + log [NH4 ] = [NH3] = 0.10 0.10 [0.28] = 9.20 Buffers A buffered solution resists changes in pH when acids and bases are added or when dilution occurs. definition - a buffer solution is composed of: a weak-acid and its salt (conjugate base) or a weak-base and its salt (conjugate acid) Buffers Henderson-Hasselbalch Equation [H3O+] = Ka ([HA]/[A-]) pH = pKa + log([A-]/[HA]) when the [A-] = [HA] pH = pKa AH HA! REMEMBER THE RESULT FROM THE a plots Biological Buffers • Biochemical reactions are especially sensitive to pH. Most biological molecules contain groups of atoms that may be charged or neutral depending on pH, and whether these groups are charged or neutral has a significant effect on the biological activity of the molecule. • In all multicellular organisms, the fluid within the cell and the fluids surrounding the cells have a characteristic and nearly constant pH. This pH is maintained in a number of ways, and one of the most important is through buffer systems. Two important biological buffer systems are the dihydrogen phosphate system and the carbonic acid system. The phosphate buffer system • • The phosphate buffer system operates in the internal fluid of all cells. This buffer system consists of dihydrogen phosphate ions (H2PO4-) as hydrogen-ion donor (acid) and hydrogen phosphate ions (HPO42-) as hydrogen-ion acceptor (base). H2PO4-(aq) H+(aq) + HPO42-(aq) Ka = [H +] [HPO42-] =6.23 × 10-8 at 25oC [H2PO4-] • If additional hydrogen ions enter the cellular fluid, they are consumed in the reaction with HPO42-, and the equilibrium shifts to the left. If additional hydroxide ions enter the cellular fluid, they react with H2PO4-, producing HPO42-, and shifting the equilibrium to the right. when the concentrations of H2PO4- and HPO42- are the same, what will the pH equal? 7.21 Buffer solutions are most effective at maintaining a pH near the value of the pKa. In mammals, cellular fluid has a pH in the range 6.9 to 7.4, and the phosphate buffer is effective in maintaining this pH range. Carbonate Buffer Another biological fluid in which a buffer plays an important role in maintaining pH is blood plasma. In blood plasma, the carbonic acid and hydrogen carbonate ion equilibrium buffers the pH. In this buffer, carbonic acid (H2CO3) is the hydrogen-ion donor (acid) and hydrogen carbonate ion (HCO3-) is the hydrogen-ion acceptor (base). H2CO3(aq) H+(aq) + HCO3-(aq) Additional H+ is consumed by HCO3- and additional OH- is consumed by H2CO3. Ka for this equilibrium is 7.9 × 10-7, and the pKa is 6.1 at body temperature. In blood plasma, the concentration of hydrogen carbonate ion is about twenty times the concentration of carbonic acid. The pH of arterial blood plasma is 7.40. If the pH falls below this normal value, a condition called acidosis is produced. If the pH rises above the normal value, the condition is called alkalosis. The concentrations of hydrogen carbonate ions and of carbonic acid are controlled by two independent physiological systems. Carbonic acid concentration is controlled by respiration, that is through the lungs. Carbonic acid is in equilibrium with dissolved carbon dioxide gas. H2CO3(aq) CO2(aq) + H2O(l) An enzyme called carbonic anhydrase catalyzes the conversion of carbonic acid to dissolved carbon dioxide. In the lungs, excess dissolved carbon dioxide is exhaled as carbon dioxide gas. CO2(aq) CO2(g) The concentration of hydrogen carbonate ions is controlled through the kidneys. Excess hydrogen carbonate ions are excreted in the urine. The much higher concentration of hydrogen carbonate ion over that of carbonic acid in blood plasma allows the buffer to respond effectively to the most common materials that are released into the blood. Normal metabolism releases mainly acidic materials: carboxylic acids such as lactic acid (HLac). These acids react with hydrogen carbonate ion and form carbonic acid. HLac(aq) + HCO3-(aq) Lac-(aq) + H2CO3(aq) The carbonic acid is converted through the action of the enzyme carbonic anhydrase into aqueous carbon dioxide. H2CO3(aq) CO2(aq) + H2O(l) An increase in CO2(aq) concentration stimulates increased breathing, and the excess carbon dioxide is released into the air in the lungs. The carbonic acid-hydrogen carbonate ion buffer works throughout the body to maintain the pH of blood plasma close to 7.40. The body maintains the buffer by eliminating either the acid (carbonic acid) or the base (hydrogen carbonate ions). Changes in carbonic acid concentration can be effected within seconds through increased or decreased respiration. Changes in hydrogen carbonate ion concentration, however, require hours through the relatively slow elimination through the kidneys EXAMPLE: What is the ratio of [H2CO3]/[HCO3-] in the blood buffered to a pH of 7.40? Ka = 4.4 x 10-7M [H3O+] = 10-pH = 10-7.4 = 4.0 x 10-8M Buffer Capacity • refers to the ability of the buffer to retard changes in pH when small amounts of acid or base are added • the ratio of [A-]/[HA] determines the pH of the buffer whereas the magnitude of [A-] and [HA] determine the buffer capacity





![[H 2 PO 4 - ] = 0.800 M.](http://s2.studylib.net/store/data/005623813_1-92875a3e2acb84ddbb79ead23a1c6630-300x300.png)



