Heat Practice Problems
advertisement
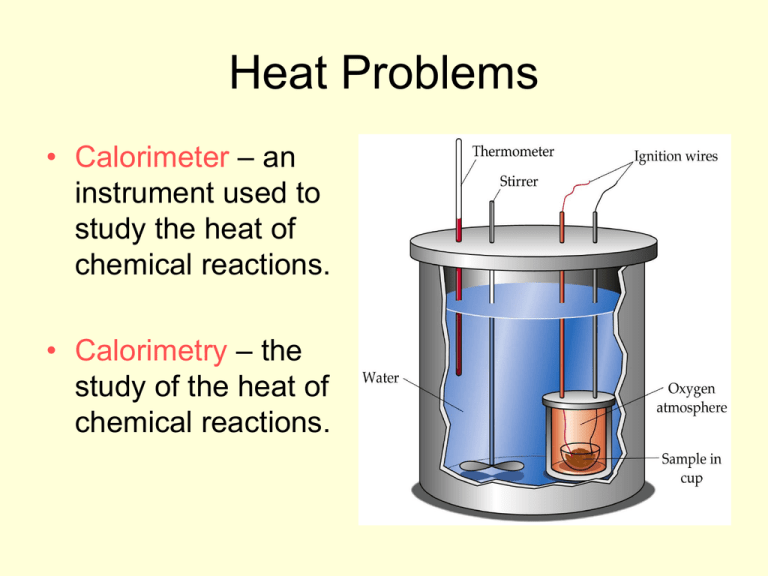
Heat Problems • Calorimeter – an instrument used to study the heat of chemical reactions. • Calorimetry – the study of the heat of chemical reactions. Problem 1 • A 1.00 g sample of octane is burned in a calorimeter containing 1200 g water at 25oC. After the reaction, the final temperature of the water is 33.2oC. How much heat did the octane release? Problem 2 • Calculate the specific heat of platinum of 1092 Joules of heat were released when 125 grams of platinum cooled 65.2 Celsius degrees. Problem 3 • What would be the final temperature if 8.94 x 103 J of heat were added to 454 grams of copper at 23oC? Problem 4 • A 50g piece of aluminum at a temperature of 100oC is placed in 50g of water at 20oC. What is the final temperature of the system? 1. (4.18 J/gC)(1200g)(33.2-25) 41131 J 2. 1092J = (C)(125g)(62.5) 0.134 J/gC 3. 8.94 x 103 = (.385J/gC)(454g)(Tf-23) 74oC 4. -[(0.9J/gC)(50g)(Tf-100)] = (4.18J/gC)(50g)(Tf-20) 34oC











