Review – Russo Chapter 1 1. Differentiate between a “personal
advertisement

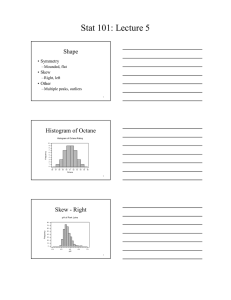
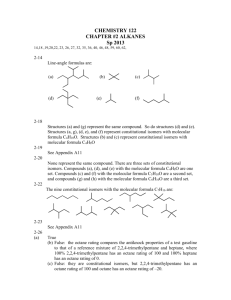
Review – Russo Chapter 1 1. Differentiate between a “personal theory” and a “scientific theory”. Personal – maybe supported by some evidence, maybe not Scientific – supported by lots of evidence 2. Circle each answer that is a compound and a pure substance: a) Sn b) CO c) KMnO4 d) H2 e) KCl + MgCl2 3. Below are listed many different properties of octane (C8H18), one of the components of gasoline. Identify these properties as physical or chemical and justify each choice. a) Octane is a liquid at room temperature. P – no new substance forms b) Octane is a volatile liquid (meaning it evaporates rather quickly at room temperature) P – no new substance forms c) Octane burns (combines with oxygen) to form carbon dioxide and water vapor. C – new substance forms d) Liquid octane is colorless. P – no new substance forms e) Octane is less dense than water (it floats on water). P – no new substance forms f) Octane is insoluble in water. P – no new substance forms g) Octane is a poison. C – new substance forms h) Liquid octane dissolves grease well. P – no new substance forms i) Octane forms octyl chloride (C8H17Cl) when combined with chlorine gas. C – new substance forms k) Octane freezes below 57˚C P – no new substance forms 5. Identify whether these are chemical reactions or not. a) Phosphorus (P4) reacts with oxygen (O2) forming diphosphorus pentoxide (P2O5). C b) When nitrogen dioxide gas is cooled, it changes from brown to colorless. not c) Cookies baking in the oven turn lightly brown. C d) Residential ozonators work by passing an electric current through the air, converting the oxygen (O2) in the air to ozone (O3). C 6. Circle all diagrams at right that show a mixture of two compounds: 7. Identify the following as heterogeneous or homogeneous mixtures: a) the contents of your backpack het b) a pizza het c) kool-aid all mixed up & ready to drink hom d) window glass hom 8. Identify all of the following that are pure elements: a) Fe b) NO2 c) C6H12O6 d) N2 & O2 e) O3 9. Is it scientifically proper to develop a theory, but not do any experiments to test that theory? Why or why not? No – theories must be supported by evidence!!! 10. Circle all diagrams at right that show a mixture of an element and a compound: 11. Given the heating curve below for sulfur, clearly label the following items in the appropriate locations (use arrows as needed to indicate direction or exact location or regions. Solid Gas Melting point Liquid Boiling Condensation Point Freezing Melting 12. As you move from point B to point C on the heating curve, what is happening to the kinetic energy of the molecules? Nothing, KE does not change 13. As you move from point D to point C on the curve, is the change endothermic or exothermic? 14. On the phase change diagram for water at the right, what are the conditions at the triple point? 0.006 atm & 0.001°C 15. What endothermic phase change occurs in water at about 0°C and 0.005 atm? Sg 16. At what pressure, if any, would ice melt at a temperature of 1°C? None – ice can only melt at temperatures of 0.001°C or colder according to the diagram.