Density - McSteiger MST
advertisement

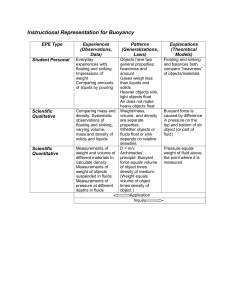
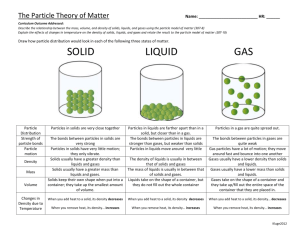
Fluids Density In the last activity, you discovered that no matter how much substance you used, the mass-to-volume ratio stayed the same. This ratio is density. Density – A measure of the amount of matter (mass) per unit volume of a substance. Time for ... Mr. Edwards The Density Song What is Density? You can use the particle theory to help explain density: ◦ Particles of a solid are usually more closely packed than particles of a liquid, which are more closely packed than particles of a gas. ◦ Therefore, solids are generally denser than liquids, which are denser than gases. Density is also affected by the type of particles a substance is made of. ◦ Oil and water are both liquids, but water is much denser. ◦ Helium is much less dense than air. Density & The Particle Theory Can you explain the science behind these experiments/demonstrations? Think of how density relates to the particle theory. ◦ Floating Egg ◦ HWK: Explain how density affects the results for each egg. Use a diagram to explain each. ◦ Floating/Sinking Coke? ◦ Underwater Volcano - Density? ◦ Ask yourself, why does this happen? Density & The Particle Theory Density is determined by the following equation: Density Mass Volume The common units of measurement for density of solids and liquids are g/mL or g/cm , and for gases, kg/m . T/P/S: Based on the formula, how would you … a) Increase density? b) Decrease density? c) Real-life examples? Calculating Density An empty container has a mass of 50g. When 75mL of oil are placed in it, the total mass is 120g. Calculate the density of oil. Given: 1. mass of container = 50g mass of container & oil = 120g Volume of oil = 75mL Need to find mass of oil first: • Mass = 120g – 50g = 70g 2. Use the formula to find density: • Density = mass/volume = 70g/75mL = 0.93g/mL • Therefore, the density of the oil is 0.9g/mL. Calculating Density Density of Some Common Materials 1. Use the particle theory to explain densities in solids, liquids, and gases. 2. Calculate the density of a diamond if the volume of the diamond is 0.50cm and its mass is 1.75g. 3. To prep for the upcoming lab, research the densities of: Water, Rubbing Alcohol, Maple Syrup, Laundry Detergent, Vegetable Oil Homework Questions Fun with Density! It’s time for you to try one of the class’ density demonstrations. Complete each demonstration, but add an additional test: ◦ Floating Eggs – Test sugar ◦ Underwater Volcano – Cold Water in Hot Water Make sure you create your own observation table to record your data. Think qualitative and quantitative data. Introduction: Seven Layer Density Lab Prep: ◦ Read over lab handout ◦ Complete a hypothesis & variables ◦ Create the necessary tables for observations Science Lab: Layer Liquid Fluid Density Observations Water Rubbing Alcohol Maple Syrup Laundry Detergent Vegetable Oil Lab Chart Example - Liquids Fluid/ Object Observations Density (Description) Air Bubble Wooden Bead Plastic Gem Bolt Popcorn Kernel Lab Chart Example – Gas & Solids