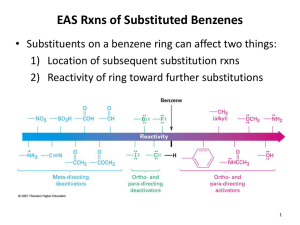
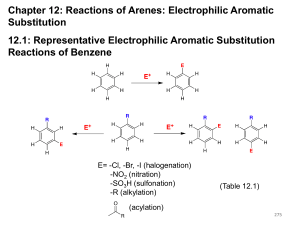
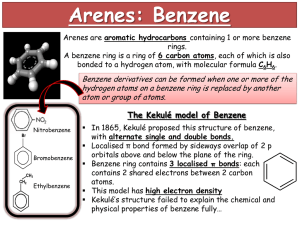
STRUCTURE OF BENZENE
advertisement

Benzene Part 1 Starter: name the functional group. 1 2 4 3 5 Learning outcomes • • • • • Define arene and aromatic Name aromatic compounds Describe the structure of benzene Review the evidence for this structure Show how electrophilic substitutions occurs with different electrophiles Arene and aromatic • Look at page 4 • Write your definition of Arene and Aromatic Benzene • Isolated by Faraday in 1825 Physical properties: • Clear, colourless, hydrocarbon • 92.3% Carbon, 7.7% hydrogen. • 0.250g was vaporised at 100oC had a volume of 98cm3 • Boiling point 80oC, melting point 5oC Calculate the empirical formula • 92.3% Carbon, 7.7% hydrogen. Element % Ar Moles Ratio empirical formula CH C 92.3 12.0 7.69 7.69 1 H 7.7 1.0 7.7 7.69 1 Calculate the molecular formula 1 mole of gas is 24dm3 at RTP • 0.250g was vaporised at 100oC had a volume of 98cm3. • From this it was calculated that the molecular mass was 78g • So what is the molecular formula? The relative molecular mass of the sample is 78 The molecular formula78/13 = 6 Therefore the molecular formula is C6H6 Molymods Find as many structures as possible for C6H6 Draw them in your notes Problem • Doesn’t react like an alkene- no reaction with HBr • Doesn’t undergo electrophilic addition • Enthalpy change should be about 800 kJmol-1, where as it is only 207kJmol-1 THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120kJ of energy is released per mole. C6H10(l) + H2(g) ——> C6H12(l) 2 3 - 120 kJ mol-1 THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120kJ of energy is released per mole. C6H10(l) + H2(g) ——> C6H12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360kJ per mole when reduced to cyclohexane Theoretical - 360 kJ mol-1 (3 x -120) C6H6(l) + 3H2(g) ——> C6H12(l) 2 3 - 120 kJ mol-1 THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120kJ of energy is released per mole. C6H10(l) + H2(g) ——> C6H12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360kJ per mole when reduced to cyclohexane Theoretical - 360 kJ mol-1 (3 x -120) C6H6(l) + 3H2(g) ——> C6H12(l) 2 Actual benzene releases only 208kJ per mole when reduced, putting it lower down the energy scale 3 - 120 kJ mol-1 Experimental - 208 kJ mol-1 THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120kJ of energy is released per mole. C6H10(l) + H2(g) ——> C6H12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360kJ per mole when reduced to cyclohexane Theoretical - 360 kJ mol-1 MORE STABLE THAN EXPECTED by 152 kJ mol-1 (3 x -120) C6H6(l) + 3H2(g) ——> C6H12(l) 2 Actual benzene releases only 208kJ per mole when reduced, putting it lower down the energy scale It is 152kJ per mole more stable than expected. This value is known as the RESONANCE ENERGY. 3 - 120 kJ mol-1 Experimental - 208 kJ mol-1 THERMODYNAMIC EVIDENCE FOR STABILITY When unsaturated hydrocarbons are reduced to the corresponding saturated compound, energy is released. The amount of heat liberated per mole (enthalpy of hydrogenation) can be measured. When cyclohexene (one C=C bond) is reduced to cyclohexane, 120kJ of energy is released per mole. C6H10(l) + H2(g) ——> C6H12(l) Theoretically, if benzene contained three separate C=C bonds it would release 360kJ per mole when reduced to cyclohexane Theoretical - 360 kJ mol-1 MORE STABLE THAN EXPECTED by 152 kJ mol-1 (3 x -120) C6H6(l) + 3H2(g) ——> C6H12(l) 2 Actual benzene releases only 208kJ per mole when reduced, putting it lower down the energy scale It is 152kJ per mole more stable than expected. This value is known as the RESONANCE ENERGY. 3 - 120 kJ mol-1 Experimental - 208 kJ mol-1 Kekulé 1865 STRUCTURE OF BENZENE HOWEVER... • it did not readily undergo electrophilic addition - no true C=C bond • only one 1,2 disubstituted product existed • all six C—C bond lengths were similar; C=C bonds are shorter than C-C • the ring was thermodynamically more stable than expected To explain the above, it was suggested that the structure oscillated between the two Kekulé forms but was represented by neither of them. It was a RESONANCE HYBRID. Next: X-ray diffraction Bond type Structure Bond length C C Cyclohexane 0.15 C C Benzene 0.14 C C cyclohexene 0.13 STRUCTURE OF BENZENE - DELOCALISATION The theory suggested that instead of three localised (in one position) double bonds, the six p (p) electrons making up those bonds were delocalised (not in any one particular position) around the ring by overlapping the p orbitals. There would be no double bonds and all bond lengths would be equal. It also gave a planar structure. 6 single bonds one way to overlap adjacent p orbitals another possibility This final structure was particularly stable and resisted attempts to break it down through normal electrophilic addition. However, substitution of any hydrogen atoms would not affect the delocalisation. delocalised pi orbital system STRUCTURE OF BENZENE Exam question • In this question, one mark is available for the quality of spelling, punctuation and grammar. • Describe with the aid of suitable diagrams the bonding and structure of a benzene molecule. Discussion of the π-bonding p-orbitals overlap (1) above and below the ring (1) (to form) π-bonds / orbitals (1) any of the first three marks are available from a labelled diagram eg bonds (π-bonds / electrons) are delocalised (1) 4 marks Other valid points – any two of: • ring is planar / • C-C bonds are equal length / have intermediate length/strength between C=C and C-C / • σ-bonds are between C-C and/or C-H • bond angles are 120° 6 MAX 2 out of 4 marks (1)(1) Quality of written communication two or more sentences with correct spelling, punctuation and grammar 1 [7] Homework • Produce a leaflet or a poster showing where benzene is used and hence why it is important. Due Friday 10th September Learning outcomes • • • • • Define arene and aromatic Name aromatic compounds Describe the structure of benzene Review the evidence for this structure Show how electrophilic substitutions occurs with different electrophiles Naming aromatic compounds Phenol Chlorobenzene Methylbenzene Many of these compounds are foul smelling and toxic, they are still called aromatic • When a long alkyl chain with other substitutions is present, think of the benzene as substituted onto the chain, using phenyl and a number to position the chain. Cl CH3– CH – CH – CH3 2-Chloro-3-phenylbutane Naming dominos Identify the following molecules as alkene, arene or cycloaklane 1. 2. 3. 4. 5. CH3CH2CH2CH2CH3 C6H5CH3 CH3CH=CHCH2CH3 CH3CH(CH3)CH2CH3 Identify the following molecules as alkene, arene, alkane or cycloalkane 1. 2. 3. 4. 5. CH3CH2CH2CH2CH3 alkane C6H5CH3 arene CH3CH=CHCH2CH3 alkene CH3CH(CH3)CH2CH3 alkane cycloalkane Learning outcomes • • • • • Define arene and aromatic Name aromatic compounds Describe the structure of benzene Review the evidence for this structure Show how electrophilic substitutions occurs with different electrophiles ELECTROPHILIC SUBSTITUTION Theory The high electron density of the ring makes it open to attack by electrophiles Addition to the ring would upset the delocalised electron system Substitution of hydrogen atoms on the ring does not affect the delocalisation Because the mechanism involves an initial disruption to the ring, electrophiles must be more powerful than those which react with alkenes Overall there is ELECTROPHILIC SUBSTITUTION ELECTROPHILIC SUBSTITUTION Theory The high electron density of the ring makes it open to attack by electrophiles Addition to the ring would upset the delocalised electron system Substitution of hydrogen atoms on the ring does not affect the delocalisation Because the mechanism involves an initial disruption to the ring, electrophiles must be more powerful than those which react with alkenes Overall there is ELECTROPHILIC SUBSTITUTION Mechanism • a pair of electrons leaves the delocalised system to form a bond to the electrophile • this disrupts the stable delocalised system and forms an unstable intermediate • to restore stability, the pair of electrons in the C-H bond moves back into the ring • overall there is substitution of hydrogen ... ELECTROPHILIC SUBSTITUTION ELECTROPHILIC SUBSTITUTION REACTIONS - NITRATION Reagents conc. nitric acid and conc. sulphuric acid (catalyst) Conditions reflux at 55°C Equation C6H6 + HNO3 ———> C6H5NO2 + H2O nitrobenzene ELECTROPHILIC SUBSTITUTION REACTIONS - NITRATION Reagents conc. nitric acid and conc. sulphuric acid (catalyst) Conditions reflux at 55°C Equation C6H6 Mechanism + HNO3 ———> C6H5NO2 + H2O nitrobenzene ELECTROPHILIC SUBSTITUTION REACTIONS - NITRATION Reagents conc. nitric acid and conc. sulphuric acid (catalyst) Conditions reflux at 55°C Equation C6H6 + HNO3 ———> C6H5NO2 + H2O nitrobenzene Mechanism Electrophile NO2+ , nitronium ion or nitryl cation; it is generated in an acid-base reaction... 2H2SO4 + acid HNO3 base 2HSO4¯ + H3O+ + NO2+ ELECTROPHILIC SUBSTITUTION REACTIONS - NITRATION Reagents conc. nitric acid and conc. sulphuric acid (catalyst) Conditions reflux at 55°C Equation C6H6 + HNO3 ———> C6H5NO2 + H2O nitrobenzene Mechanism Electrophile NO2+ , nitronium ion or nitryl cation; it is generated in an acid-base reaction... 2H2SO4 + acid Use HNO3 base 2HSO4¯ + H3O+ + NO2+ The nitration of benzene is the first step in an historically important chain of reactions. These lead to the formation of dyes, and explosives. ELECTROPHILIC SUBSTITUTION REACTIONS - HALOGENATION Reagents chlorine and a halogen carrier (catalyst) Conditions reflux in the presence of a halogen carrier (Fe, FeCl3, AlCl3) chlorine is non polar so is not a good electrophile the halogen carrier is required to polarise the halogen Equation C6H6 + Cl2 ———> C6H5Cl + HCl Mechanism Electrophile Cl+ it is generated as follows... Cl2 + FeCl3 a Lewis Acid FeCl4¯ + Cl+ Now your turn Write the mechanisims for the following electrophiles: 1. CH3+ 2. CH3CO+ Where does the electrophile end up? Groups with +I Groups with a - I Mostly 2, 4 and 6 positions Mostly with 3 and 5 positions OH Cl CH3 COOH NH2 NO2 + I effect - I effect Learning outcomes • • • • • Define arene and aromatic Name aromatic compounds Describe the structure of benzene Review the evidence for this structure Show how electrophilic substitutions occurs with different electrophiles Diploma students only FRIEDEL-CRAFTS REACTIONS OF BENZENE - ALKYLATION Overview Alkylation involves substituting an alkyl (methyl, ethyl) group Reagents a halogenoalkane (RX) and anhydrous aluminium chloride AlCl3 Conditions room temperature; dry inert solvent (ether) Electrophile a carbocation ion R+ (e.g. CH3+) Equation C6H6 + C2H5Cl ———> C6H5C2H5 + HCl Mechanism General A catalyst is used to increase the positive nature of the electrophile and make it better at attacking benzene rings. AlCl3 acts as a Lewis Acid and helps break the C—Cl bond. FRIEDEL-CRAFTS REACTIONS OF BENZENE - ALKYLATION Catalyst anhydrous aluminium chloride acts as the catalyst the Al in AlCl3 has only 6 electrons in its outer shell; a LEWIS ACID it increases the polarisation of the C-Cl bond in the haloalkane this makes the charge on C more positive and the following occurs RCl + AlCl3 AlCl4¯ + R+ FRIEDEL-CRAFTS REACTIONS - INDUSTRIAL ALKYLATION Industrial Alkenes are used instead of haloalkanes but an acid must be present Phenylethane, C6H5C2H5 is made by this method Reagents ethene, anhydrous AlCl3 , conc. HCl Electrophile C2H5+ Equation C6H6 + C2H4 Mechanism the HCl reacts with the alkene to generate a carbonium ion electrophilic substitution then takes place as the C2H5+ attacks the ring Use ethyl benzene is dehydrogenated to produce phenylethene (styrene); this is used to make poly(phenylethene) - also known as polystyrene (an ethyl carbonium ion) ———> C6H5C2H5 (ethyl benzene) FRIEDEL-CRAFTS REACTIONS OF BENZENE - ACYLATION Overview Acylation involves substituting an acyl (methanoyl, ethanoyl) group Reagents an acyl chloride (RCOX) and anhydrous aluminium chloride AlCl3 Conditions reflux 50°C; dry inert solvent (ether) Electrophile RC+= O Equation C6H6 + CH3COCl ( e.g. CH3C+O ) ———> C6H5COCH3 + HCl Mechanism Product A carbonyl compound (aldehyde or ketone) FURTHER SUBSTITUTION OF ARENES Theory It is possible to substitute more than one functional group. But, the functional group already on the ring affects... • how easy it can be done Group • where the next substituent goes ELECTRON DONATING Example(s) Electron density of ring Ease of substitution Position of substitution OH, CH3 Increases Easier 2,4,and 6 ELECTRON WITHDRAWING NO2 Decreases Harder 3 and 5