electron
advertisement
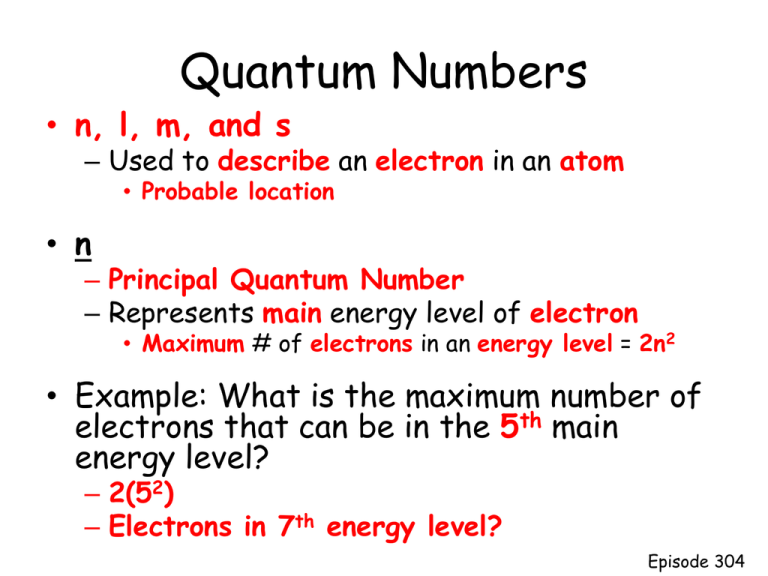
Quantum Numbers • n, l, m, and s – Used to describe an electron in an atom • Probable location • n – Principal Quantum Number – Represents main energy level of electron • Maximum # of electrons in an energy level = 2n2 • Example: What is the maximum number of electrons that can be in the 5th main energy level? – 2(52) – Electrons in 7th energy level? Episode 304 Quantum Numbers • l • The 2nd quantum number – Angular momentum quantum number • Describes the orbital shape within an energy level • Number of orbital shapes possible in an energy level = n http://www.youtube.com/watch?v=KjNgq16jEY&list=PLD1C287B0E1484083&index=9&feature=plcp Episode 304 Orbital Shapes • • • • • Designated s, p, d, and f Level 1: s Level 2: s, p Level 3: s, p, d Level 4: s, p, d, f Episode 304 How many electrons can each sublevel hold? • • • • s = 1 orbital x 2 e-/orbital = p = 3 orbitals x 2 e-/orbital = d = 5 orbitals x 2 e-/orbital = f = 7 orbitals x 2 e-/orbital = Energy Level E sublevel n Type of orbital 1 2 2 e6 e10 e14 e- # of orbitals # e- in orbital # e- = 2n2 s s 1 1 2 2(12) = 2 p 3 6 2 2(22) = 8 Episode 304 Quantum Numbers • m • The 3rd quantum number – Magnetic Quantum Number • Describes the orientation of the orbital in space Episode 304 Quantum Numbers • s • The 4th quantum number – Spin Quantum Number http://www.youtube.com/watch?v=2ypC7rnFXLU&list=PLD1C287B0 E1484083&index=1&feature=plcp • Describes the spin of the electron in orbital • Ground state • Lowest energy arrangement of electrons – Aufbau Principle Episode 304 Diagonal Rule Examples: • Hydrogen – 1 electron – 1s1 • Lithium – 3 electrons – 1s2 2s1 • Nitrogen – 7 electrons – 1s2 2s2 2p3 Episode 304 • Electron Configurations – Describes the electron distribution within an atom • Longhand electron configuration – Nitrogen 1s2 2s2 2p3 • Orbital Notation – Uses arrows to represent electrons • Examples: – Hydrogen 1s1 1s Episode 304 Nitrogen • 1s2 2s2 2p3 1s 2s 2p • Hund’s Rule • Orbitals of equal energy are each occupied by one electron before any orbital is occupied by a second electron (spinning in opposite direction) • Pauli Exclusion Principle • No two electrons in the same atom can have the same set of 4 quantum numbers Episode 304