CHE 333 Class 6
advertisement

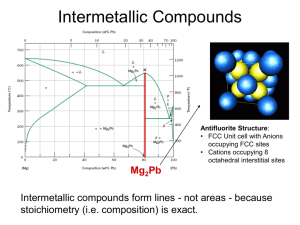
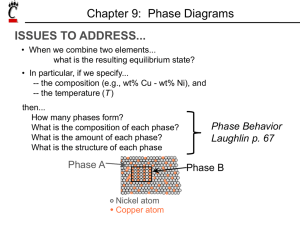
Class 6 Phase Diagrams And Microstructure Homeworks • If everyone agrees, homeworks will be in the lab on a bench in alphabetical groups. • Placed there Wednesday so can be collected after 1.00pm • Lab schedule starts Wed 2.00pm room 121 Crawford Hall. • NOTE First Exam move to Oct 10th • Multiple Choice – eg how does sodium and chlorine bond? Microstructures and Composition At eutectic composition, and temp, amount of a = 97.5-61.9/97.5-19.2 = 35.6/78.3= 45% amount of b = 61.9-19.2/97.5-19.2 = 42.7/78.3= 55% For 70% Pb 30% Sn, a phase if formed in a+liquid range, called pro eutectic a. Amount of proeutectic a and eutectic a+b is obtained from inverse lever arm rule Amount of pro eutectic a = 61.9-30.0/61.9-19.2 =31.9/42.7=0.75=75% Amount of eutectic a+b = 30.0-19.5/61.9-19.2 = 10.5/42.4 = 0.25 = 25% The composition of the a will be the same independent of whether it is pro eutectic or eutectic at 80.8%Pb 19.2% Sn. Lead Tin Microstructures 90 %Pb 10%Sn 70% Pb 30%Sn 38.1%Pb 61.9% Sn 50%Pb 50%Sn Lead Tin Microstructures 15%Pb 85%Sn Equiaxed Single Phase Grain Structure Iron Carbon (Fe3C) Phase Diagram Primary solid solubility in a is 0.022wt% C, for is 2.14 wt% C. Eutectoid reaction is important for steels. > a + Fe3C at 727C. At 726C , a 99.978%Fe 0.022%C Fe3C 93.3%C 6.7%C Fe3C is a compound and a Metastable phase, not an equilibrium phase, but stable unless thermally changed. Eutectoid composition Is Fe 99.24%, C 0.76% Eutectoid Composition At 727C Eutecoid Reaction occurrs. > a + Fe3C Above 727C all , equiaxed. Below 727C, a + Fe3C Amounts of a and Fe3 C given by Inverse Lever Arm Rule. Amount of a = 6.7 – 0.76/ 6.7-0.022 = 5.94/6.678 = 0.89 = 89% a Amount of Fe3C = 0.76- 0.022/ 6.7-0.022 = 0.738 / 6.678 = 0.11 = 11% Fe3C a composition is 99.978%Fe, 0.022 C Fe3C composition if 93.3%Fe, 6.7%C Eutectoid Formation a Carbon moves from a Fe3C Iron moves from carbide Growth Direction Eutectoid Alternate ferrite and cementite plate structure in Eutectoid steel. Hypereutecoid Strucutres Hypereutecoid – more carbon then eutectoid >0.76% Start in single phase austenite, , range, grains of . Cool into two phase region, then some transforms to Fe3C on the existing grain boundaries. Called “Pro Eutectoid” cementite. composition follows phase boundary to the eutectiod composition, so at 727C the remaining transforms to pearlite, the mixture of a + Fe3C. Cementite exists as Pro eutectoid and eutectoid forms. Hypoeutectoid Steels Hypoeutecoid steels – less carbon than eutectoid < 0.76% Start in single phase austenite, , range, grains of . Cool into two phase region, then some transforms to a on the existing grain boundaries. Called “Pro Eutectiod” ferrite. composition follows phase boundary to the eutectiod composition, so at 727C the remaining transforms to pearlite, the mixture of a + Fe3C. Ferrite exists as Pro eutectoid and eutectoid forms.( Pearlite has narrow plates in this case of a steel with 0.08%C) Homeworks. 1. 2. From the PbSn phase diagram, for a 70% Pb, 30%Sn alloy, at what temperature does solid first form. At the eutectic temperature, how much proeutectic phase is present and how much eutectic phase? What are the compositions at 100oC? For a steel containing 0.4% C:a) At what temperature does austenite start to transform on cooling from 950oC? b) How much proeutectoid phase is present at the eutectoid temperature c) What is its composition?