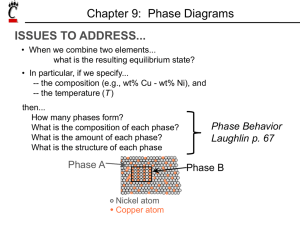
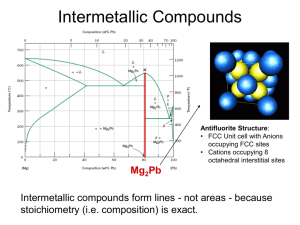
Phase Equilibrium
advertisement

Phase Equilibrium Engr 2110 – Chapter 9 Dr. R. R. Lindeke Topics for Study Definitions of Terms in Phase studies Binary Systems Complete solubility (fully miscible) systems Multiphase systems Eutectics Eutectoids and Peritectics Intermetallic Compounds The Fe-C System Some Definitions System: A body of engineering material under investigation. e.g. Ag – Cu system, NiO-MgO system (or even sugar-milk system) Component of a system: Pure metals and or compounds of which an alloy is composed, e.g. Cu and Ag or Fe and Fe3C. They are the solute(s) and solvent Solubility Limit: The maximum concentration of solute atoms that may dissolve in the Solvent to form a “solid solution” at some temperature. Definitions, cont Phases: A homogenous portion of a system that has uniform physical and chemical characteristics, e.g. pure material, solid solution, liquid solution, and gaseous solution, ice and water, syrup and sugar. Single phase system = Homogeneous system Multi phase system = Heterogeneous system or mixtures Microstructure: A system’s microstructure is characterized by the number of phases present, their proportions, and the manner in which they are distributed or arranged. Factors affecting microstructure are: alloying elements present, their concentrations, and the heat treatment of the alloy. Figure 9.3 (a) Schematic representation of the one-component phase diagram for H2O. (b) A projection of the phase diagram information at 1 atm generates a temperature scale labeled with the familiar transformation temperatures for H2O (melting at 0°C and boiling at 100°C). Figure 9.4 (a) Schematic representation of the one-component phase diagram for pure iron. (b) A projection of the phase diagram information at 1 atm generates a temperature scale labeled with important transformation temperatures for iron. This projection will become one end of important binary diagrams, such as that shown in Figure 9.19 Definitions, cont Phase Equilibrium: A stable configuration with lowest free-energy (internal energy of a system, and also randomness or disorder of the atoms or molecules (entropy). Any change in Temperature, Composition, and Pressure causes an increase in free energy and away from Equilibrium thus forcing a move to another ‘state’ Equilibrium Phase Diagram: It is a “map” of the information about the control of microstructure or phase structure of a particular material system. The relationships between temperature and the compositions and the quantities of phases present at equilibrium are represented. Definition that focus on “Binary Systems” Binary Isomorphous Systems: An alloy system that contains two components that attain complete liquid and solid solubility of the components, e.g. Cu and Ni alloy. It is the simplest binary system. Binary Eutectic Systems: An alloy system that contains two components that has a special composition with a minimum melting temperature. With these definitions in mind: ISSUES TO ADDRESS... • When we combine two elements... what “equilibrium state” would we expect to get? • In particular, if we specify... --a composition (e.g., wt% Cu - wt% Ni), and --a temperature (T ) and/or a Pressure (P) then... How many phases do we get? What is the composition of each phase? How much of each phase do we get? Phase B Phase A Nickel atom Copper atom Gibb’s Phase Rule: a tool to define the number of phases and/or degrees of phase changes that can be found in a system at equilibrium F CPN where: F is # degrees of freedom of the system (independent parameters) C is # components (elements) in system P is # phases at equil. N is # "noncompostional" parameters in system (temp &/or Pressure) For any system under study the rule determines if the system is at equilibrium For a given system, we can use it to predict how many phases can be expected Using this rule, for a given phase field, we can predict how many independent parameters (degrees of freedom) we can specify Typically, N = 1 in most condensed systems – pressure is fixed! Looking at a simple “Phase Diagram” for Sugar – Water (or milk) Effect of Temperature (T) & Composition (Co) • Changing T can change # of phases: See path A to B. • Changing Co can change # of phases: See path B to D. B (100°C,70) D (100°C,90) 1 phase watersugar system Adapted from Fig. 9.1, Callister 7e. Temperature (°C) 100 2 phases L 80 (liquid) 60 L (liquid solution i.e, syrup) 40 20 0 0 + S (solid sugar) A (20°C,70) 2 phases 20 40 60 70 80 100 Co =Composition (wt% sugar) Phase Diagram: A Map based on a System’s Free Energy indicating “equilibuim” system structures – as predicted by Gibbs Rule • Indicate ‘stable’ phases as function of T, P & Composition, • We will focus on: -binary systems: just 2 components. -independent variables: T and Co (P = 1 atm is almost always used). T(°C) • 2 phases are possible: 1600 Phase Diagram -1500 for Cu-Ni system L (liquid) a (FCC solid solution) L (liquid) 1400 1300 a (FCC solid solution) 1200 An “Isomorphic” Phase System 1100 1000 0 20 40 60 80 • 3 ‘phase fields’ are observed: L L+a a 100 wt% Ni Phase Diagrams: • Rule 1: If we know T and Co then we know the # and types of all phases present. A(1100°C, 60): 1 phase: a B(1250°C, 35): 2 phases: L + a 1600 L (liquid) B (1250°C,35) • Examples: T(°C) 1500 1400 1300 1200 1100 1000 Cu-Ni phase diagram a (FCC solid solution) A(1100°C,60) 0 20 40 60 80 100 wt% Ni Phase Diagrams: • Rule 2: If we know T and Co we know the composition of each phase • Examples: T(°C) Cu-Ni system A TA Co = 35 wt% Ni tie line 1300 L (liquid) At T A = 1320°C: Only Liquid (L) B TB CL = Co ( = 35 wt% Ni) a At T D = 1190°C: (solid) 1200 D Only Solid ( a) TD Ca = Co ( = 35 wt% Ni) 20 3032 35 4043 50 At T B = 1250°C: CLCo Ca wt% Ni Both a and L adapted from Phase Diagrams CL = C liquidus ( = 32 wt% Ni here) of Binary Nickel Alloys, P. Nash (Ed.), ASM International, Materials Park, OH, 1991. Ca = C solidus ( = 43 wt% Ni here) Figure 9.31 The lever rule is a mechanical analogy to the massbalance calculation. The (a) tie line in the two-phase region is analogous to (b) a lever balanced on a fulcrum. The Lever Rule Tie line – a line connecting the phases in equilibrium with each other – at a fixed temperature (a so-called Isotherm) How much of each phase? We can Think of it as a lever! So to balance: T(°C) tie line 1300 L (liquid) B TB a (solid) 1200 R 20 Ma ML S 30C C 40 C a L o R 50 S M a S M L R wt% Ni WL C C0 ML S a ML M a R S Ca CL Wa C CL R 0 R S Ca CL Therefore we define • Rule 3: If we know T and Co then we know the amount of each phase (given in wt%) • Examples: Cu-Ni system T(°C) Co = 35 wt% Ni A TA At T A : Only Liquid (L) W L = 100 wt%, W a = 0 At T D: Only Solid ( a) W L = 0, Wa = 100 wt% At T B : Both a and L WL S 43 35 73 wt % R + S 43 32 Wa R = 27 wt% R +S 1300 TB 1200 TD 20 tie line L (liquid) B R S D 3032 35 CLCo a (solid) 40 43 50 Ca wt% Ni Notice: as in a lever “the opposite leg” controls with a balance (fulcrum) at the ‘base composition’ and R+S = tie line length = difference in composition limiting phase boundary, at the temp of interest Ex: Cooling in a Cu-Ni Binary • Phase diagram: Cu-Ni system. • System is: --binary i.e., 2 components: Cu and Ni. T(°C) L (liquid) 130 0 L: 35 wt% Ni a: 46 wt% Ni • Consider Co = 35 wt%Ni. Cu-Ni system A 35 32 --isomorphous i.e., complete solubility of one component in another; a phase field extends from 0 to 100 wt% Ni. L: 35wt%Ni B C 46 43 D 24 L: 32 wt% Ni 36 120 0 a: 43 wt% Ni E L: 24 wt% Ni a: 36 wt% Ni a (solid) 110 0 20 30 Adapted from Fig. 9.4, Callister 7e. 35 Co 40 50 wt% Ni Cored vs Equilibrium Phases • Ca changes as we solidify. • Cu-Ni case: First a to solidify has Ca = 46 wt% Ni. Last a to solidify has Ca = 35 wt% Ni. • Fast rate of cooling: Cored structure • Slow rate of cooling: Equilibrium structure First a to solidify: 46 wt% Ni Last a to solidify: < 35 wt% Ni Uniform C a: 35 wt% Ni Cored (Non-equilibrium) Cooling Notice: The Solidus line is “tilted” in this nonequilibrium cooled environment Binary-Eutectic (PolyMorphic) Systems has a ‘special’ composition with a min. melting Temp. Cu-Ag T(°C) system 2 components Ex.: Cu-Ag system 1200 • 3 single phase regions L (liquid) (L, a, ) 1000 a L+a • Limited solubility: L+ 779°C 800 T a: mostly Cu E 8.0 71.9 91.2 : mostly Ag 600 • TE : No liquid below TE a 400 • CE : Min. melting TE composition 200 • Eutectic transition L(CE) 0 a(CaE) + (CE) 20 40 60 CE 80 Co , wt% Ag 100 EX: Pb-Sn Eutectic System (1) • For a 40 wt% Sn-60 wt% Pb alloy at 150°C, find... --the phases present: a + T(°C) --compositions of phases: CO = 40 wt% Sn Ca = 11 wt% Sn C = 99 wt% Sn --the relative amount of each phase (by lever rule): C - CO S = Wa = R+S C - Ca Pb-Sn system 300 200 L (liquid) a L+ a 18.3 150 61.9 R 97.8 S a+ 100 99 - 40 59 = = 67 wt% 99 - 11 88 C - Ca W = R = O C - Ca R+S L+ 183°C = = 40 - 11 29 = = 33 wt% 99 - 11 88 0 11 20 Ca 40 Co Adapted from Fig. 9.8, Callister 7e. 60 80 C, wt% Sn 99100 C EX: Pb-Sn Eutectic System (2) • For a 40 wt% Sn-60 wt% Pb alloy at 220°C, find... --the phases present: a + L T(°C) --compositions of phases: CO = 40 wt% Sn Ca = 17 wt% Sn CL = 46 wt% Sn --the relative amount of each phase: CL - CO 46 - 40 = Wa = CL - Ca 46 - 17 6 = = 21 wt% 29 Pb-Sn system 300 a 220 200 L+a R L (liquid) L+ S 183°C a+ 100 0 CO - Ca 23 = WL = = 79 wt% CL - Ca 29 17 20 Ca 40 46 60 Co CL C, wt% Sn 80 100 Microstructures In Eutectic Systems: I • Co < 2 wt% Sn • Result: T(°C) L: Co wt% Sn 400 L a --at extreme ends 300 --polycrystal of a grains i.e., only one solid phase. L a 200 L+ a (Pb-Sn System) a: Co wt% Sn TE a+ 100 0 Co 10 20 30 Co, wt% Sn 2% (room Temp. solubility limit) Microstructures in Eutectic Systems: II L: Co wt% Sn • 2 wt% Sn < Co < 18.3 wt% Sn 400T(°C) • Result: Initially liquid Then liquid + a then a alone finally two solid phases a polycrystal fine -phase inclusions L L a 300 L+a a 200 TE a: Co wt% Sn a 100 a+ 0 10 20 Pb-Sn system 30 Co Co , wt% 2 (sol. limit at T room ) 18.3 (sol. limit at TE) Sn Microstructures in Eutectic Systems: III • Co = CE • Result: Eutectic microstructure (lamellar structure) --alternating layers (lamellae) of a and crystals. T(°C) L: Co wt% Sn 300 Pb-Sn system TE a 200 L+ a L a 0 L 183°C 100 20 18.3 40 Micrograph of Pb-Sn eutectic microstructure : 97.8 wt% Sn a: 18.3 wt%Sn 60 CE 61.9 80 100 97.8 C, wt% Sn 160 m 45.1% a and 54.8% -- by Lever Rule Lamellar Eutectic Structure Microstructures in Eutectic Systems: IV • 18.3 wt% Sn < Co < 61.9 wt% Sn • Result: a crystals and a eutectic microstructure L: Co wt% Sn T(°C) L a L 300 Pb-Sn system a 200 a L L+a R TE L+ S S R primary a eutectic a eutectic 0 20 18.3 40 60 61.9 Ca = 18.3 wt% Sn CL = 61.9 wt% Sn Wa = S = 50 wt% R+S WL = (1- Wa) = 50 wt% • Just below TE : a+ 100 • Just above TE : 80 Co, wt% Sn 100 97.8 Ca = 18.3 wt% Sn C = 97.8 wt% Sn Wa = S = 72.6 wt% R+S W = 27.4 wt% Hypoeutectic & Hypereutectic Compositions 300 L T(°C) a 200 L+ a L+ TE a+ (Pb-Sn System) 100 0 20 40 hypoeutectic: Co = 50 wt% Sn from Metals Handbook, 9th ed., Vol. 9, Metallography and Microstructures, American Society for Metals, Materials Park, OH, 1985. a a a 60 80 eutectic 61.9 Co, wt% Sn hypereutectic: (illustration only) eutectic: Co = 61.9 wt% Sn a a a 175 m 100 160 m eutectic micro-constituent “Intermetallic” Compounds Adapted from Fig. 9.20, Callister 7e. Mg2Pb An Intermetallic Compound is also an important part of the Fe-C system! Note: an intermetallic compound forms a line - not an area because stoichiometry (i.e. composition) is exact. Eutectoid & Peritectic – some definitions Eutectic: a liquid in equilibrium with two solids L cool heat a+ Eutectoid: solid phase in equilibrium with two solid phases S2 S1+S3 intermetallic compound - cementite cool heat a + Fe3C (727ºC) Peritectic: liquid + solid 1 in equilibrium with a single solid 2 (Fig 9.21) S1 + L S2 +L cool heat (1493ºC) Eutectoid & Peritectic Peritectic transition + L Cu-Zn Phase diagram Eutectoid transition + Adapted from Fig. 9.21, Callister 7e. Iron-Carbon (Fe-C) Phase Diagram 1600 1200 a + Fe3C +L (austenite) 1000 a 800 600 120 m Result: Pearlite = alternating layers of a and Fe3C phases (Adapted from Fig. 9.27, Callister 7e.) S +Fe3C 727°C = Teutectoid R S 1 0.76 L+Fe3C R B 400 0 (Fe) A 1148°C 2 3 a+Fe3C 4 5 6 Fe3C (cementite) L 1400 L + Fe3C -Eutectoid (B): Max. C solubility in iron = 2.11 wt% T(°C) C eutectoid • 2 important points -Eutectic (A): 6.7 4.30 Co, wt% C Fe3C (cementite-hard) a (ferrite-soft) Hypoeutectoid Steel T(°C) 1600 L +L 1200 (austenite) 1000 a a a 800 1148°C 727°C r s aRS a + Fe3C 1 C0 w pearlite = w 0.76 400 0 (Fe) pearlite w a =S/(R+S) w Fe3 =(1-w a ) C L+Fe3C + Fe3C w a =s/(r +s) 600 w =(1- wa ) a (Fe-C System) pearlite 2 3 4 5 6 Fe3C (cementite) 1400 Adapted from Figs. 9.24 and 9.29,Callister 7e. (Fig. 9.24 adapted from Binary Alloy Phase Diagrams, 2nd ed., Vol. 1, T.B. Massalski (Ed.-inChief), ASM International, Materials Park, OH, 1990.) 6.7 Co , wt% C 100 m Hypoeutectoid steel proeutectoid ferrite Hypereutectoid Steel T(°C) 1600 L Fe3C +L 1200 (austenite) 1000 r 800 w Fe3C =r/(r +s) w =(1-w Fe3C ) a R 600 400 0 (Fe) pearlite 1148°C L+Fe3C +Fe3C 0.76 (Fe-C System) s S 1 Co w pearlite = w w a =S/(R+S) w Fe3C =(1-w a ) a +Fe3C 2 3 4 5 6 Fe3C (cementite) 1400 adapted from Binary Alloy Phase Diagrams, 2nd ed., Vol. 1, T.B. Massalski (Ed.-in-Chief), ASM International, Materials Park, OH, 1990. 6.7 Co , wt%C 60 mHypereutectoid steel pearlite proeutectoid Fe3C Example: Phase Equilibria For a 99.6 wt% Fe-0.40 wt% C at a temperature just below the eutectoid, determine the following: a) b) c) composition of Fe3C and ferrite (a) the amount of carbide (cementite) in grams that forms per 100 g of steel the amount of pearlite and proeutectoid ferrite (a) Solution: CO = 0.40 wt% C Ca = 0.022 wt% C CFe3 C = 6.70 wt% C a) composition of Fe3C and ferrite (a) b) the amount of carbide (cementite) in grams that forms per 100 g of steel 1600 Fe3C Co Ca x100 1400 Fe3C a CFe 3C Ca T(°C) Fe3C 5.7 g a 94.3 g +L L+Fe3C 1148°C (austenite) 1000 Fe C (cementite) 0.4 0.022 x 100 5.7g 6.7 0.022 1200 L + Fe3C 800 727°C R S a + Fe3C 600 400 0 Ca CO 1 2 3 4 Co , wt% C 5 6 6.7 CFe 3C Solution, cont: c) the amount of pearlite and proeutectoid ferrite (a) note: amount of pearlite = amount of just above TE C Ca o x 100 51.2 g a C Ca pearlite = 51.2 g proeutectoid a = 48.8 g 1600 L 1400 T(°C) 1200 1000 + Fe3C 800 600 727°C RS a + Fe3C 1 Ca CO C 2 11.1% Fe3C (.111*51.2 gm = 5.66 gm) & 88.9% a (.889*51.2gm = 45.5 gm) total a = 45.5 + 48.8 = 94.3 gm L+Fe3C 1148°C (austenite) 400 0 Looking at the Pearlite: +L 3 4 Co , wt% C 5 6 Fe C (cementite) Co = 0.40 wt% C Ca = 0.022 wt% C Cpearlite = C = 0.76 wt% C 6.7 Alloying Steel with More Elements Ti Mo Si W Cr Mn Ni wt. % of alloying elements Adapted from Edgar C. Bain, Functions of the Alloying Elements in Steel, American Society for Metals, 1939, p. 127.) • Ceutectoid changes: Ceutectoid (wt%C) T Eutectoid (°C) • Teutectoid changes: Ni Cr Si Ti Mo W Mn wt. % of alloying elements Adapted from Edgar C. Bain, Functions of the Alloying Elements in Steel, American Society for Metals, 1939, p. 127.) Looking at a Tertiary Diagram When looking at a Tertiary (3 element) P. diagram, like this image, the Mat. Engineer represents equilibrium phases by taking “slices at different 3rd element content” from the 3 dimensional Fe-C-Cr phase equilibrium diagram From: G. Krauss, Principles of Heat Treatment of Steels, ASM International, 1990 What this graph shows are the increasing temperature of the euctectoid and the decreasing carbon content, and (indirectly) the shrinking phase field Fe-C-Si Ternary phase diagram at about 2.5 wt% Si This is the “controlling Phase Diagram for the ‘Graphite‘ Cast Irons Summary • Phase diagrams are useful tools to determine: -- the number and types of phases, -- the wt% of each phase, -- and the composition of each phase for a given T and composition of the system. • Alloying to produce a solid solution usually --increases the tensile strength (TS) --decreases the ductility. • Binary eutectics and binary eutectoids allow for (cause) a range of microstructures.