Local Anesthetics
advertisement

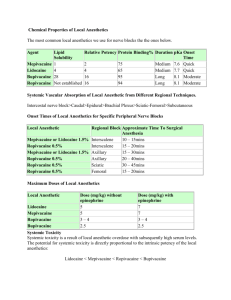
PHARMACOLOGY OF REGIONAL OPHTHALMIC ANESTHESIA Berrin Gunaydin, MD, PhD Gazi University Scool of Medicine Department of Anesthesiology Ankara, Turkey www.berringunaydin.com OUTLINE • Overview of pharmacology of commonly used local anesthetics and adjuvants for ophthalmic regional anesthesia • Efficacy of these drugs with regard to improving akinesia, analgesia, speed of onset and reducing block failure 2 Chronology of Amide Local Anesthetic Development Agent Initial investigator Date • Cocaine C17H21NO 4 Carl Koller, an ophthalmology trainee tookNiemann cocaine orally and noticed 1860 • numbness Benzocaine in his tongueC9H11NO2 Salkowski 1895 Koller and Gartner reported topical cocaine anesthesia of the eye in Procaine C13H20N2O2 Einhorn 1904 animals and human (1884) Dibucaine C20H29N3O2 Meischer 1925 Tetracaine C15H24N2O2 Eisler 1928 Etidocaine C17H28 N202 Lidocaine C14H22N2O Löfgren, Lundquist 1943 Clorprocaine C13H19CIN2O2 Marks, Rubin 1952 Mepivacaine C15H22N2O Ekenstam 1957 Bupivacaine C18H28N2O Ekenstam 1963 Prilocaine C13H20N2O Lofgren 1959 Articaine C13H20N2O3S Rusching 1969 Ropivacaine Levobupivacaine Adams,Kronberg, Takman C17H26N2O (Butterworth J. Clinical Pharmacology of Local Anesthetics) Ekenstam&Sandberg Ekenstam&others 1972 1996 1999 I.LOCAL ANESTHETICS (LA) Chemical structure Aromatic ring-intermediate chain-amino group • Ester linkage-COO • Amid linkage-NHCO Veering B. Local Anesthetics 4 Properties of local anesthetics • • • • • • • Ionization Lipid solubility Protein binding Chirality Mechanism of action Metabolism and elimination Toxicity 5 Physicochemical properties of local anesthetics pKa PB (%) (25C) Onset time Potency Duration ESTERS Cocaine 8.7 Slow 98 High Long Procaine 8.9 Slow 6 Low Short Lipid solubility Potency Amethocaine 8.5 Slow 76 Medium Medium AMIDES Lidocaine 7.7 Fast 64 Medium Medium Prilocaine 7.8 Fast 55 Medium Medium Mepivacaine 7.6 Fast 75 Medium Medium Etidocaine 7.7 Fast 94 High Long Bupivacaine 8.1 Medium 95 High Long Ropivacaine 8.2 Medium 94 Medium Long Levobupivacaine 8.1 Medium 96 High Long Protein binding Duration of action pKa Onset time 6 Bupivacaine, Etidocaine, Mepivacaine, Prilocaine, Ropivacaine Have asymmetric carbon molecule AYNA Levobupivacaine, Ropivacaine are chiral 7 • Bupivacaine & prilocaine contain chiral carbon • Both have R and S configuration (racemic) • Cocaine, naturally original LA, is a pure levarotatory enantiomer (-cocaine) • Dextrorotatory cocaine (-cocaine or pseudococaine) • Stereospecificty has not been investigated until bupivacaine cardiotoxicity R (+) bupivacaine has a much longer dwell time in cardiac sodium channels than the S(-) form. Of additional signficance more potent depressant effect on brain-stem cardiorespiratory neurons of R(+) bupivacaine compared with its S(-) enantiomer De Jong RH. Local Anesthetic Pharmacology 9 Mechanism of action 10 Metabolism • Ester type local anesthetics are split in plasma by pseudocholinesterase • Primary metabolic product is p-aminobenzoic acid (PABA) which is highly allergenic • Plasma half-life significantly prolongs in case of deficiency or presence of atypical pseudocholinesterase • Since amide type local anesthetics are metabolized in the liver, only 1-3% can be seen in the urine 11 Elimination • Ester local anesthetics are almost entirely eliminated in plasma by ester breakdown except cocaine • Amide local anesthetics except prilocaine are metabolized in liver (>90%) – Lidocaine and etidocaine have high extraction rate (elimination depends primarily on liver perfusion) – Bupivacaine and mepivacaine have limited hepatic extraction rate – Prilocaine has a high elimination rate (considerably eliminated outside the liver) 12 Elimination ESTERS Local anesthetics Cocaine Procaine Amethocaine AMIDES Lidocaine Prilocaine Mepivacaine Bupivacaine Ropivacaine Levobupivacaine Elimination Metabolism ESTERS Local anesthetics Metabolism-breakdown products Benzil-ekgonin, Ekgonin metil ester, Ekgonin Cocaine Procaine PABA, in plasma by pseudocholinesterase Dietil amino etanol p-butil amino benzoik asit Amethocaine AMIDES Lidocaine in liver by CP450 (CYP1A2 & CYP3A4 at low and high %, respectively) Mono ethy glysi xsilid (MEGX) Glysin xyilid (GX) Prilocaine O-tolidin, Nitrozotolidin Mepivacaine Oksopipekolo-ksilid, CH3oksopipekolo-ksilid Bupivacaine Desbutil-bupivakain Hidroksi-bupivakain Ropivacaine OH-pipekoloksilid (PPX) 3 ,4 OH-ropivakain Levobupivacaine Desbutil-bupivakain Hidroksi-bupivakain Toxicity of ESTER TYPE LA Cocaine ester of benzoic acid, excellent topical anesthetic (4-10%), only LA producing vasoconstriction at clinical concentrations, high potential for systemic toxicity Procaine Derivative of PABA, weak LA, slow onset, short duration of action, low potency and rapid plasma hydrolysis lead to low systemic toxicity but hydrolization to PABA may cause allergic reactions after repeated use Amethocaine Butyl aminobenzoic acid derivative of procaine, potent ,long acting, hydrolysis by plasma cholinesterase (slower than procaine), potential for systemic toxicity is HIGH Veering B. Local Anesthetics 15 Toxicity of AMIDE TYPE LA Lidocaine Most versatile, commonly used,rapid onset of action,moderate duration of action prolongs with epinephrine, safely used for all types of local anesthesia, potential for systemic toxicity is INTERMEDIATE Prilocaine toluidine derivative tertiary amine,clinical profile similar to lidocaine, LEAST TOXIC amino-amide LA, significant methemoglobinemia can occur >10 mg/kg Mepivacaine Structurally related to lidocaine, rapid onset of action, duration of action is somewhat longer than lidocaine, epinephrine prolongs duration of action by 75%, potential for systemic toxicity is SIMILAR TO LIDOCAINE Etidocaine Structurally similar to lidocaine, faster onset of action and similar duration of action when compared to bupivacaine, LESS TOXIC than other long acting LA due to its greater distribution and clearance, not in current practice Bupivacaine Homologue of mepivacaine, greater anesthetic potency, prolonged duration of action, onset of analgesia is slow, MORE CARDIOTOXIC than equipotent doses of lidocaine Ropivacaine S-enantiomer of bupivacaine, long acting LA, LESS ARRHYTHMOGENIC than bupivacaine Levobupivacaine 16 Veering B. Local Anesthetics 17 Local Toxicity – Neurototxicity (direct injection to nerve) rarely occurs when local anesthetics used alone for ophthalmic anesthesia, however it can happen with vasoconstrictors and high orbital pressures – Myotoxicity (direct injection to muscle) to m.inferior oblique and rectus during inferotemporal injection and to m.rectus medial during medial cantus injection 18 Systemic Toxicity – Central Nerve System (CNS) – With increased local anesthetic doses seizures may arise in the amygdala – Further local anesthetic dosing leads to CNS excitation progressing to CNS depression and eventual respiratory arrest – Cardiovascular System (CVS) Cardiovascular collapse Coma Convulsions Myoclonic jerks Tremors Garrulousness Circumoral numbness Omnius feelings Tinnitus, Vertigo Metalic …. 19 II.ADJUVANTS • Hyaluronidase • Vasoconstrictors - Epinephrine • Alkalinization (pH adjustment with sodium bicarbonate) • Others 20 Hyaluronidase I • Enzyme that reversibly liquefies the interstitial barrier by depolimerization of the hyaluronic acid to tetrasaccharide • 5-150 IU/mL (15 IU/mL in UK) • Available as a powder in LA solution • Orbital swelling due to rare allergic reactions or excessive doses and orbital pseudotumour 21 Hyaluronidase II • Addition of hyaluronidase to mixture of lidocaine+bupivacaine decrease onset time during retrobulbar anesthesia • Addition of both epinephrine and hyaluronidase to pH-adjusted bupivacaine prolongs the duration of action during peribulbar block Nicoll et al. Anesth Analg1986 Zahl et al. Anesthesiology 1990 Vasoconstrictors I • Optimal concentration of epinephrine is 1:200000 (5 µg/mL) • Recommended dose 3-5 µg/kg • Absorption of the local anesthetic is reduced • Thus, avoids high concentrations of LA in the plasma • Allows higher dose administration Vasoconstrictors II • Increase duration of block particularly short acting LA • Minimize bleeding from small vessels • May cause vasoconstriction of the ophthalmic artery compromising retinal circulation • Epinephrine containing solutions should be avoided in elderly suffering from cerebrovascular and cardiovascular diseases Epinephrine • Addition of epinephrine to lidocaine and mepivacaine markedly prolongs the duration of action (in addition to the vasocontriction and physochemical properties like local binding or intrinsic vasoactivity may contribute) • However, addition of epinephrine to prilocaine, etidocaine (hardly prolongs the duration of action), and bupivacaine (is relatively small) Alkalinization • Local anesthetics penetrate nerve cell membranes in non-ionized form and intracellularly in their ionized form • Addition of sodium bicarbonate to LA (which are weak bases) increases their pH thus decreasing the ionized/nonionized ratio • 30-50% reduction in onset time • Extent and quality of block improved Recommended doses for avoiding precipitation Lidocaine, Prilocaine or Mepivacaine 9 mL + 1 mL 8.4% NaHCO3 Bupivacaine,Levobupivacaine or Ropivacaine 9.9 mL + 0.1 mL 8.4% NaHCO3 Short acting LA Lidocaine Prilocaine Mepivacaine Bupivacaine Ropivacaine Levobupivacaine Others – Clonidine • Mhajed et al, Reg Anesth 1996 • Connely et al. Reg Anesth Pain Med 1999 – Temperature • Onset time decreases for all LA at body temperature – Mixture • Lidocaine-bupivacaine-hyaluronidase-epinephrinevecuronium Reah et al. Anaesthesia 1998 Preservatives Preserves the stability of LA drugs in solution • PABA (such as methy/ethyl or propyl paraben) • Metabisulfite (sodium bisulfite) • Ethylendiaminetetraacetate (EDTA) PABA – Parabens are aliphatic esters of PABA – Sodium benzoat and benzoic acid are not chemically parabens but close relation to structure might cause crossreactivity with parabens – Inhibit growth of fungi and yeast (less antibacterial) in multidose vials – However, all parabens have been removed from the contemporary formulations, currently packaged as singledose vials – Of importance, ester based LA drugs like procaine, 2chloroprocaine or tetracaine structurally related to PABA can be metabolized to PABA derivatives (30% + skin reaction) DiFazio and Rowlingson. Additives to local anesthetic soutions. MacPherson Pharmaceutics for the anaesthetist. Anaesthesia 2001 • Metabusulfite (sodium • EDTA bisulfite) • added 2• An antioxidant to prevent chloroprocaine breakdown of epinephrine instead of metabisulfit in LA solution containing is also potentially epinephrine neurotoxic secondary • Provides greater stability to chelation of and shell life (usually pH4.5) calcium ions in • In case of low pH, this paraspinal muscles preservative leads to leading to severe formation of SO2 and sulfurous acid (neurotoxic) muscle spasms DiFazio and Rowlingson. Additives to local anesthetic soutions. Adverse effects due to additives • Patients at risk – Children, especially neonates – Patienst receiving TPN – Patients receiving long term parenteral therapy – Patients in ICU – Patients suffering from chronic pain with indwelling pump systems MacPherson Pharmaceutics for the anaesthetist. Anaesthesia 2001 Levobupivakain • Bupivakain’den daha mı az toksik? • Bupivakain kadar potent mi? • Bupivakain’in yerini alabilir mi? 34 Peribulber anestezi Di Donato et al. Efficacy and comparison of 0.5% levobupivacaine with 0.75% ropivacaine for peribulbar anaesthesia in cataract surgery. Eur J Anaesthesiol 2006 • • • • 208 hasta,katarakt operasyonu % 0.5 Levo-6 mL % 0.75 Ropivakain-6 mL Levobupivakain ile duyu ve motor bloğun başlaması daha erken bitmesi daha geç 35 Peribulber anestezi Magalhaes et al.Racemic bupivacaine, levobupivacaine and ropivacaine in regional anesthesia for ophthalmology- a comparative study. Rev Assoc Med Bras 2004 • • • • • 97 hasta, katarakt cerrahisi 7 mL, % 0.75 Bupi, Levo, Ropi Benzer anestezik etkinlik Göziçi basıncına etki benzer Hastaların yaşlı olması ve yüksek volüm kullanılması nedeniyle Levo ve Ropi daha uygun 36 37 Side Effects Lokal anestezik ilaca bağlı • Toksisite – SSS – KVS – Nörotoksisite (Lidokain, klorprokain) • Allerji • Methemoglobinemi ( Prilokain) Eklenen vazokonstriktöre bağlı Yönteme bağlı 38 Cardiovascular System • Hızlı Na+ kanallarının blokajı – İletimde yavaşlama – QRS kompleksinde genişleme ve PQ intervalinde uzama – AV blok ve aritmiler • Kardiyak mitokondriyal enerji metabolizmasında blokaj • SSS aracılı kardiyak disritmiler 39 Cardiotoxic effect İki aşamalıdır • Önce sempatik aktivasyon ile taşikardi, HT • Sonra aritmi ve kardiyak depresyon • Bupivakain>Levobupivakain>Ropivakain 40 Cardiotoxicity Bupivacaine • Na kanallarından yavaş ayrıldığından selektif kardiyak etkileri var • Kalpte elektriksel iletiyi baskılar • Ventriküler aritmilere zemin hazırlar • Elektromekanik disosiasyona yol açar 41 Sodyum mmol/L Osmolality pH H+ mmol/L Bupivakain % 0.25 133 272 6.96 109 Bupivakain % 0.5 134 287 6.74 182 Bupivakain % 0.75 125 281 6.57 269 Ropivakain % 0.2 143 292 6.82 152 Ropivakain % 0.5 126 287 6.65 222 Levobupivakain % 0.25 149 308 6.42 379 Levobupivakain % 0.5 151 322 6.04 914 Levobupivakain % 0.75 151 334 5.85 1413 İV Levobupivakain Anesth Analg 1999 • Epidural anestezi sırasında yanlışlıkla 19 mL % 0.75 Levo iv enjeksiyonu • Konuşma bozukluğu, eksitasyon • Nöbet ve KVS bulguları yok • 10 dk sonra plazma düzeyi 2.7 µg/mL • Bupivakain için toksik doz 2 - 4 µg/mL Levobupivakain-SSS toksisitesi • Kortikal ve subkortikal düzeylerde nöronal desenkronizasyon • Santral inhibitör yolağın bloğu • KVS toksik belirtilerinden önce oluşur – Levobupivakain ile bupivakainden daha az nörotoksisite – Hayvan çalışmalarında konvülsiyona yol açan doz (mg/kg) bupivakain’den %40 daha fazla Levobupivakain-SSS toksisitesi • Gönüllülerde bir çalışma • 40 mg intravenöz bupivakain veya levobupivakain – Bupivakain %91 SSS semptomları – Levobupivakain %64 SSS semptomları Nimmo W, Sanderson B. ESA abstract 1988 Levobupivakain-KVS toksisitesi • Tüm hayvan çalışmalarında bupivakainle karşılaştırıldığında üstün kardiyak güvenlik profili • Yüksek dozlarda aritmi insidansı – Bupivakain’den 4 misli daha az – 3.4 kez daha kısa süreli – Kendiliğinden düzelir Levobupivakain-KVS toksisitesi Levobupivakain ve bupivakain’in KVS etkileri – gönüllülerde İV verilim Barsley H, et al. Br J Clin Pharmacol 1998 Levobupivakain-KVS toksisitesi • • • • • 63 y, prostatektomi, genel anestezi Yanlışlıkla IV 125 mg Levobupivakain 5 dk sonra hipotansiyon, hafif bradikardi (55/dk) Adrenalin bolus, noradrenalin inf Operasyon sorunsuz tamamlanmış! SSS-KVS etkileri karşılaştırması • 13 gönüllü • Intravenöz infüzyon – Levobupivakain – Ropivakain • 10 mg/dk – SSS semptomları görülene dek • ECG, CO, MAP and KAH Stewart J et al. Anesth Analg 2003 Lokal Anestezikler Metabolize Edildiği Yer • • • • • • • • • • • • • • • • • Metabolitleri Kokain Plazma esterazları (???) Benzil-ekgonin, Ekgonin metil ester, Ekgonin Ester Prokain Para amino benzoik asit(PABA), Dietil amino etanol Kloro-prokain Kloro amino benzoik asit Tetrakain p-butil amino benzoik asit Lidokain Glisin ksilid Amid Artikain(Kartikain) Mepivakain Prilokain Nitrozotolidin Bupivakain Hidroksi-bupivakain Ropivakain 3 ,4 OH-ropivakain Levobupivakain Hidroksi-bupivakain Mono etil glisin ksilid Karaciğer (??) Artikainik asit Oksopipekolo-ksilid, Metil oksopipekolo-ksilid O-tolidin, Desbutil-bupivakain OH-pipekoloksilid Desbutil-bupivakain