Local anaesthetics

LOCAL
ANAESTHETICS
DR.SUDHIR
MUBARAK AL KABEER HOSPITAL
DEFINITION
They are defined as drugs that can produce reversible inhibition of excitation
& conduction in perpipheral nerve fibres & nerve endings & thus produce a loss of sensation in a circumscribed area of the body
HISTORY
Captain James cook – puffer fishtetrodotoxin – 18 th century
1884 – Cocaine – Freud & Koller – used first for corneal anaesthesia
1905 – Procaine – Einhorn – first synthetic local anaesthetic
1943- Lidocaine – Lofgren
Chemical Structure
Physical Properties (structure)
Ester :
Amide
:
Example:
R 3
R 4
R 3
R 4
Changes to any part of the molecule lead to alterations in activity & toxicity
Increases in the length of the intermediate alcohol group, up to a critical length , result in greater anaesthetic potency
Beyond this critical length, increased toxicity results
Compounds with an ethyl ester, such as procaine, exhibit the least toxicity
The length of the two terminal groups on the tertiary amino-N group are similarly important
The addition of a butyl group to mepivacaine results in bupivacaine, which differs by, a. increased lipid solubility & protein binding b. greater potency c. a longer duration of action
ESTERS
COCAINE
PROCAINE
CHLORPORCAINE
TETRACAINE
(AMETHOCAINE)
BENZOCAINE
AMIDES
LIDOCAINE
PRILOCAINE
MEPIVACAINE
BUPIVACAINE
ROPIVACAINE
ETIDOCAINE
CINCHOCAINE
(DIBUCAINE)
NEURONAL TRANSMISSION
SODIUM & POTASSIUM
CHANNELS
Voltage sensitive sodium channel
It sorrounds aqueous pore
Three subunitsα β
1
β
2
α unit –largest- MW 260 kDa
It’s a single long peptide containing four hydrophobic regions – I,II,III,IV
They are connected to each other by intracellular bridges
Each region concists of six membrane spanning segments( S1- S6)
S4 segment is the voltage sensor
The intracellular bridge between III & IV is the
Inactivation gate
This gate is responsible for occluding Na+ channel & hence inactivation.
Potassium channel is similar but with 4 subunits- 14 types of k+ channels are present
Acid base considerations related to mode of action
Rules
Acid drugs – become more NON ionized in acidic pH
Basic drugs – become more NON ionized in basic pH (alkaline pH)
Acid Drug Basic Drug
Acid pH
Environment
NON-ionized IONIZED
Basic pH
Environment
IONIZED NON-ionized
Rules
Hydrophilic = Ionized molecules (charged)
Lipophilic = Non-ionized molecules (non charged)
Lipophilic molecules penetrate membranes
Hydrophilic molecules dissolve in water
Depending if a molecule is ionized or not determines if it can pass through a cell membrane
Ionized Molecule
Non-Ionized Molecule
Basic Drug
Basic drugs – become more NON ionized in basic pH
I
N
I N I N I pH 2 pH 6 pH 8 pH 9
What’s the pKa for this drug?
pH = pK
= Ionized molecules a
+ log base acid
MODE OF ACTION
Most LA are tertiary amine Bases(B) which are administered as water soluble hydrochlorides(B.Hcl).
After injection ,the base is liberated by Alkaline pH of the ECF
B.HCL + HCO
3
= B + H
2
CO
3
+ CL -
So the LA is present in both ionised (BH + ) & non –ionised forms(B) in tissues
The proportion of each depends on Pka of drug and the ph medium into which it is adimistered
The non ionised base( B) only can diffuse through the nerve sheath,perinural tissues & neurilemma to reach neuroplasm
In the neuroplasm it gets again ionised by H + ions
B + H + = BH +
This BH + the sodium channel
They are believed to interact with phenyalanine & tyrosine residues in the S6 segment of region IV
Thus they block Na+ channels and prevent depolarisation ( Phase 0)
Benzocaine Membrane expansioncausing swelling of lipoprotein matrix of Na+ channel .
Tetrodotoxin & saxitoxindirectly block Na+ channel from the exterior of the membrane,close to the external pore
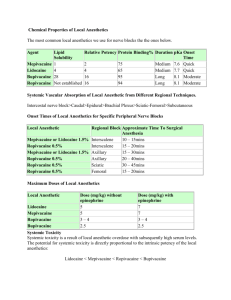
PHYSIOCHEMICAL PROPERTIES
LIPID SOLUBILITY
TISSUE PROTEIN BINDING
pKa
STEREO SPECIFICITY
VOSODILATATION
MINIMUM CONCENTRATION
FREQUENCY DEPENDANT BLOCKADE
DIFFERENTIAL BLOCKADE
LIPID SOLUBILITY
There is a close relation between lipid solubility and potency
Bupivicaine is approx 4 times more potent than lidocaine because of lipid solubility
Etidocaine(5000) > Bupivacine(4000)>
Ropivacaine>tetracine> lidocaine(150)> prilocaine=mepivacine> procaine (1)
Tissue protein binding
It primarily affects the Duration of LA
Etidocaine(96%) > Bupivacine(95%)>
Ropivacaine(94%)>mepivacine>tetracine> lidocaine(65%)> prilocaine> procaine (6%)
pKa Value- disscociation constant
This is the most important factor determining the rapidity and onset of action
Low pKa – more non-ionised- rapid onset
Procaine (8.9)> Tetracaine(8.5) >
Bupivacine(8.1) = Ropivacaine> lidocaine = prilocaine = etidocaine(7.7)>
Mepivacaine(7.6)
High pKa value – better differential blockade
Onset time & Duration
Agent
(
Onset mins )
Duration Effect of
Adreanaline
X 1 Bupivacaine 20-30 8-9 hrs
Chlorprocaine Fast in 3% 30-60 mins
Etidocaine
Lidocaine
Mepivacaine
Prilocaine
Ropivacaine
15-20
20
20
20
20
6-8 hrs
60 mins
2 hrs
2 hrs
8-9 hrs
X 1.5
X 4
X 2
X 2
X 1
STEREO SPECIFICITY
Most ester LA are (procaine,chlorprocaine,) are achiral compounds
Most amides are chiral drugs ( exception- lidocaine )
Most used clinically are Recemic mixtures
But recently s-enantiomers are produced(S-bupivacinechirocaine)
They have enhanced vasoconstricion
So longer acting
But can cause less duration & intensity of block
Also has less cardiotoxicity
Frequency or use dependent blocakde
Local anesthetics block trains of action potentials
>> single action potentials
The more frequent the A.P, the more often Na+ channels are open.
Also, affinity of the binding site increases.
Magnitude and rate of block increase
Depolarized membranes are more sensitive to local anesthetic action
The more depolarized the membrane, the more
Na+ channels are open
Differential nerve blocakde
LA cause differential nerve block
pain > temperature > touch > deep pressure > motor
Small diameter, unmyelinated C fibers
(pain and autonomic) and Aδ fibers (pain) blocked before large diameter, myelinated
Aα, Aβ or Aγ nerves (motor, limb position)
Factors Causing Differential Nerve Block
Critical length
– About 3 nodes of Ranvier need to be blocked to completely prevent a.p. conduction down a nerve
The larger the fiber, the greater the distance between nodes and the larger the area that needs to be exposed to LA
Inhibition of >70% of Na+ channels will reduce the a.p. size
Progressive blockade as you move down the axon results in failure to conduct after a sufficient length is exposed to drug
Pain fibers and sympathetic fibers fire in high frequency bursts, whereas motor fibers fire at lower frequencies.
LA with high use dependence (bupivacaine) therefore provide a better preferential block of pain and autonomic over motor function
Axons at the periphery of a nerve (outside) are blocked more readily than those in the core because they are exposed to higher concentrations
Proximal tissues are blocked more readily because their nerves lie in the perimeter of the nerve bundle
In large mixed nerves, motor fibers lie on the outside
Minimum concentration(
Cm
)
It is the minimum concentration of LA necessary to produce conduction blockade of nerve impulses
It is analogous to MAC
Larger nerve fibres – more Cm
An increased tissue pH or high frequency nerve stimulation will decrease Cm
vasodilatation
Cocaine – vasoconstrictor
( moffett’s solution)
cocaine 10% 1 ml + adrenaline 1ml
1:1000 + NaH(CO
3
)
2
2ml 8.4%.
Vosodilatation
Pro >pri> l > M > B > R
S-isomers less vasodilataion
SYSTEMIC ABSORPTION
It depends on
Dose
Vasoconstrictor presence
Site of injection
Intercostal block > caudal > paracervical> epidural> brachial plexus > intrathecal
PLASMA PROTEIN BINDING &
PLACENTAL TRANSFER
Esters are not significantly protein bound(5-10% or less)
Amides are highly protein bound by α
1 glycoprotein
-acid
There order is
B>R>M>L>Pri
In Pregnancy there is increased sensitivity to LA
High protein bound drugs have low UV:M ratio
For bupivacaine 0.2( so less transfer)
For prilocaine 0.5 ( sp more transfer)
PHARMACKOKINETICS
Plasma concentration declines in a Biexponential manner
Rapid distribution phase( 1- 3 mins) brain,myocardium,lungs,liver
Followed by slower decline phase( muscle & fat)
Terminal half life of most ester anaesthetics is short- 10 mins due to rapid hydrolysis by plasma
& tissue cholinesterases
The amides half life range from 100 mins -200 mins
Volume of distribution> total body water
Plasma clearance is comparable with liver blood flow
Low cardiac output and hepatic cirrhosis will decrease there clearance
METABOLISM & ELIMINATION
Ester metabolism
Most esters broken down by esterase enzymes
Procaine- PABA – diethyamino ethanol – diethyglycine
Exceptionally, COCAINE is resistant to hydrolysis by ChE.
It is metabolised in liver
Metabolites- Norcocaine, Ecgonine, benzoylated analouges
They may be responsible for stumulant effects on CNS
Amide metabolism
Extensively metabolised in LIVER by
AMIDASES
Prilocaine- rapidly metabolised in liver and some extent in kidney & lung
Its principle metabolite are N-Propylamine & o-
Toludine – Methaemoglibinmia(> 600 mg)
S-prilocaine produces less o-Toludine
Bupivacaine- low hepatic clearance- slowly metabolised in liver
Pipecolic acid & pipeco xylidine – metabolites
Convulsive properties
Lidociane dealkylated
Monoethy Glycine-xylidine + acetaldehyde hydrolysed
N-Ethyl Glycine + 2,6 Xylidine
4-OH- 2,6 Xylidine
(urine)
• Glycine-Xylidine, a minor metabolite is a CNS depressant – has long half life
Pulmonary Extraction
Pulmonary extraction from the venous circulation limits the amount of local anesthetic (lidocaine , bupivacaine ), & prilocaine (Citanest)) that will reach the systemic circulation
Bupivacaine : dose-dependent, first pass extraction (saturable, uptake)
Propranolol inhibits bupivacaine extraction
Propranolol reduces lidocaine & bupivacaine plasma clearance
DRUGS
COCAINE
Has central & Peripheral effects
Both effects are caused by inhibition of uptake1 in central & peripheral nerve endings
Tachycardia , arrhthmias, vosoconstriction, pupillary dilatation & other sympathomimetic effects
Corneal anaesthesia
Nasal anaesthesia ( Moffets solution)
C.I with TCA’s , pressor drugs
Dependence & abuse
Procaine
Procainamide – not broken down by ChE.
So its used for its antiarrhythmic property Class
Ia – increases APD
Vosodilator – Rx of vascular spasm caused by inadverdent intra-arterial injection
Cardioplegia- CPB
Benzocaine
( does not ionise, acts by ME)
Topical ,ear drops,ointments
Lidocaine
Most commonly used LA
Dose limits : the maximum recommended doses in the adult are, a. plain ~ 3 mg/kg b. with adrenaline ~ 7 mg/kg
2-4% - gels,ointments,creams
10 % - sprays
1-2 % epidural
Hyperbaric solutions( dextrose) 5% - Intrathecal
Hypobaric solutions( water)
Bupivacaine
0.25% to 0.75%
0.5% heavy ( hyperbaric) most commonly used drug in UK intrathecally.
the maximum recommended doses in the adult are,
a. plain ~ 2 mg/kg
b. with adrenaline ~ 2 mg/kg
NB: this equates to ~ 25 ml of 0.5% in a 70 kg adult
Chlorprocaine
Used mostly in USA
Used in OBS practice- 3% solution
Differs from procaine by additon of chlorine atom , so its
4 times more quickly hydrolysed & has more rapid onset
Preservative – neurotoxic
Ropivacaine similar to bupivacaine, less cardiotoxic
Etidocaine
Amide derived from lidocaine
It produces a more profound effect on motor than sensory nerves
TOXICITY
Toxicity
1.Caused by overdosage
2.As a part of Therapeutic procedure
3.Caused by Added vasoconstrictor
4.Specific Effects
overdosage
CNS TOXICITY
Biphasic effects
Small dose (2-4 mg/kg) will have anticonvulsant effects-Rx status epilepticus
Large dose will cause numbeness of tongue & mouth,light headedness,visual disturbances,slurring of speech,muscular twitching & tremors,restlessness & irrational conversation
Grand mal convulsions can occur( 10 mics/ml-lidocaine , 2 mics/ml – bupivacaine)
Hypoxia & acidosis potentiates convulsions
Prociane is relatively free from convulsant activity
Initial effects- depression of inhibitory cortical pathways
Profound effects- cortical & medullary depression
CVS TOXICITY
It follows CNS toxicity
Hypotension, bradycardia, bradyarrhythmias, & cardiac arrest
Bupivacine is more carditoxic
It acts on K + & Ca 2+ channels in addtion to Na + channels.
Myocardial conduction is depressed
Widening of QRS complex & distortion of ST- segemnts
High doses can cause ventricular arrhythmia
Tachycardia can enhance frequency dependent block of cardiac sodium channels & cause more cardiotoxicity
S-bupivacaine & S-Ropivacaine have less carditoxicity
As a part of theraupatic procedure
Intercostal block > caudal > paracervical> epidural> brachial plexus > intrathecal
Caused by vasoconstrictor
Sympathomimetic amines will cause cardiac arrhythmias and hypertension
Should not be added to digital nerve block,penile block .
Specific effects
Allergic responses- currently rare
Skin rash
Anaphylactic reactions
Esters – PABA – more often cause this
PABA antagonises sulphonamides
Methaemoglobinaemia – o-toludine
ADDITIVES & MIXTURES
pH adjustment – NaOH & HCl
Tonicity- NaCl
Baricity- Glucose & H
2
O
Preservatives – methyhydroxy benzoate
( fungicide)
Reducing agents – Sodium Metabisulphate
- to prevent oxidation of adrenaline
ADDITIVES & MIXTURES
vasoconstrictors
Slows the rate of absorption
Prolongs duration
Reduces toxicity
Enhnances the intensity of block
Contraindicated:
End arteries- ring blocks, penile blocks
IVRA
Adrenaline :
Most commonly used & potent
Conc: 1 in 80,000(12.5µg/ml) to
1 in 300,000(3.3µg/ml)
Max dose should not exceed 0.5 mg.
Noradrenaline :
1 in 80,000 were used but now rarely used due to its pressor effects
Felypressin :
Non catecholamine vasoconstrictor- vasopressin analouge, causes only peripheral vosoconstriction,no action on heart
Safer for IHD patients but can cause pallor constrict coronary circulation.
Dental use – 0.03 i.u./ml
Phenyehrine
Not used now , less effective than adrenaline
CO
2
(cabonated solutions)
It is supposed to speed the onset of action
CO
2 rapidly diffuses across neurilemma & decreases intracellular pH ,so enhances the conversion of tertairy base(B) to the active form(BH + )
But in clinical practice ,doubtful.
CO
2 is rapidly buffered by intracellular proteins and these solutions are unstable & can precipitate
Dextrans :
Prolong the duration because of its high molecular weight & are effective with combination with adreanline
“Macromolecules” are formed between dextrans and LA & they are held in tissues for long periods.
Hyaluronidase :
It aids in spreading LA by breaking down tissue bariers
Used mostly in Opthal practice.
Mixtures-compounding
Lidocaine + Bupivacaine
To cause faster onset with long duration
Will decrease there individual doses and thus reduce toxicity
However ‘ toxicity is additve’ if it occurs.
If ester is combined with amide, toxicity may increase because amide slows hydrolysis of ester by inhibiting plasma cholinesterase
Eutectic mixture
The combined melting point of the eutectic mixture is less than the either of the drugs
EMLA- 5% cream
Mixture of unionised(base) 2.5% prilocaine &
2.5% lidocaine
It takes 1-2 hours to act- pediatrics
Infants –contraindicated- methaemoglobinemia
TAC solution
The combination of tetracaine , adrenaline , and cocaine (TAC) has commonly been used for repair of lacerations in the face and scalp of children
Other topicals
PRAMOXINE :
Minor burns ,pruritis, sigmidoscopy, laryngoscopy
DYCLONINE
HEXYLCAINE
PIPERCOLINE
All can be used before direct laryngoscopy and they are not amides or esters ,so useful for patients allergic to them.
CLINICAL USES
Regional anaesthesia
Topical or surface anaesthesia
Local infiltration
Peripheral nerve blocks
Bier block
Epidural
Spinal
Anlagesia (propofol)
To reduce intubation response
To decrease ICT
Ventricular dysrhytmias
Suppression of grand mal seizures