General Anesthetics - Department of Pharmacology

Local/Regional Anesthetics
Michael H. Ossipov, Ph.D.
Department of Pharmacology
General concepts
•Cocaine isolated from Erythroxylon coca plant in Andes
•Von Anrep (1880) discovers local anesthetic property, suggests clinical use
•Koller introduces cocaine in opthalmology
•Freud uses cocaine to wean Karl Koller off morphine
•Halstead demonstrates infiltration anesthesia with cocaine
•Rapidly accepted in dentistry
General concepts
• Halstead (1885) shows cocaine blocks nerve conduction in nerve trunks
• Corning (1885) demonstrates spinal block in dogs
• 1905: Procaine (NOVOCAINE) synthesized
– analog of cocaine but without euphoric effects, retains vasoconstrictor effect
– Slow onset, fast offset, ester-type (allergic reactions)
General concepts
• First “modern” LA (1940s): lidocaine
(lignocaine in UK; XYLOCAINE)
– Amide type (hypoallergenic)
– Quick onset, fairly long duration (hrs)
– Most widely used local anesthetic in US today, along with bupivacaine and tetracaine
General concepts
• Cause transient and reversible loss of sensation in a circumscribed area of the body
– Very safe, almost no reports of permanent nerve damage from local anesthetics
• Interfere with nerve conduction
• Block all types of fibers (axons) in a nerve
(sensory, motor, autonomic)
Local anesthetics: Uses
• Topical anesthesia (cream, ointments, EMLA)
• Peripheral nerve blockade
• Intravenous regional anesthesia
• Spinal and epidural anesthesia
• Systemic uses (antiarrhythmics, treatment of pain syndromes)
Structure
•All local anesthetics are weak bases. They all contain:
•An aromatic group (confers lipophilicity)
diffusion across membranes, duration, toxicity increases with lipophilicity
•An intermediate chain, either an ester or an amide; and
•An amine group (confers hydrophilic properties)
– charged form is the major active form
Structure
•Formulated as HCl salt (acidic) for solubility, stability
•But, uncharged (unprotonated N) form required to traverse tissue to site of action
•pH of formulation is irrelevant since drug ends up in interstitial fluid
•Quaternary analogs, low pH bathing medium suggests major form active at site is cationic, but both charged and uncharged species are active
Mepivicaine
Etidocaine
Articaine
Lidocaine
Prilocaine
Bupivicaine
Procaine
PKa
7.6
7.7
7.8
7.9
7.9
8.1
9.1
% RN at PH
7.4
40
33
29
25
25
18
2
Onset in minutes
2 to 4
2 to 4
2 to 4
2 to 4
2 to 4
5 to 8
14 to 18
O
COCH
2
CH
2
C
2
H
5
N H
C
2
H
5
Cationic acid
Log
Base
Acid
= pH – p K a
(Henderson-Hasselbalch equation)
For procaine (p K a
= 8.9) at tissue pH (7.4)
Base
Acid
= 0.03
O
COCH
2
CH
2
N
C
2
H
5
+ H
+
C
2
H
5
Nonionized base [1.0]
Lipoid barriers (nerve sheath)
Extracellular fluid
Base Acid [1.0]
Nerve membrane
Axoplasm
*
Base Acid
[3.1]
[2.5]
Structure
Structure
Mode of action
• Block sodium channels
• Bind to specific sites on channel protein
• Prevent formation of open channel
• Inhibit influx of sodium ions into the neuron
• Reduce depolarization of membrane in response to action potential
• Prevent propagation of action potential
Mode of action
Mode of action
Mode of action
Sensitivity of fiber types
• Unmyelinated are more sensitive than myelinated nerve fibers
• Smaller fibers are generally more sensitive than large- diameter peripheral nerve trunks
• Smaller fibers have smaller “critical lengths” than larger fibers (mm range)
• Accounts for faster onset, slower offset of local anesthesia
• Overlap between block of C-fibers and A d
-fibers.
Choice of local anesthetics
• Onset
• Duration
• Regional anesthetic technique
• Sensory vs. motor block
• Potential for toxicity
Clinical use
Esters
Procaine Slow
Chloroprocaine Fast
Tetracaine Slow
Amides
Lidocaine
Mepivacaine
Fast
Fast
Bupivacaine
Ropivacaine
Etidocaine
Onset Duration
Short
Short
Long
Moderate
Moderate
Moderate Long
Moderate Long
Fast Long
Choice of local anesthetics
Technique
Topical
Appropriate drugs
Cocaine, tetracaine, lidocaine
Infiltration Procaine, lidocaine, mepivacaine, bupivacaine, ropivacaine, etidocaine
Peripheral nerve block Chloroprocaine, lidocaine, mepivacaine, bupivacaine, ropivacaine, etidocaine
Spinal
Epidural
Procaine, tetracaine, lidocaine, bupivacaine
Chloroprocaine, lidocaine, bupivacaine, ropivacaine, etidocaine
I.V. regional anesthesia Lidocaine
Factors influencing anesthetic activity
• Needle in appropriate location (most important)
• Dose of local anesthetic
• Time since injection
• Use of vasoconstrictors
• pH adjustment
• Nerve block enhanced in pregnancy
Redistribution and metabolism
• Rapidly redistributed
• More slowly metabolized and eliminated
• Esters hydrolyzed by plasma cholinesterase
• Amides primarily metabolized in the liver
Local anesthetic toxicity
• Allergy
• CNS toxicity
• Cardiovascular toxicity
Allergy
• Ester local anesthetics may produce true allergic reactions
– Typically manifested as skin rashes or bronchospasm. May be as severe as anaphylaxis
– Due to metabolism to ρ-aminobenzoic acid
• True allergic reactions to amides are extremely rare.
Systemic toxicity
• Results from high systemic levels
• First symptoms are generally CNS disturbances (restlessness, tremor, convulsions) - treat with benzodiazepines
• Cardiovascular toxicity generally later
CNS symptoms
• Tinnitus
• Lightheadedness, Dizziness
• Numbness of the mouth and tongue, metal taste in the mouth
• Muscle twitching
• Irrational behavior and speech
• Generalized seizures
• Coma
Cardiovascular toxicity
• Depressed myocardial contractility
• Systemic vasodilation
• Hypotension
• Arrhythmias, including ventricular fibrillation
(bupivicaine)
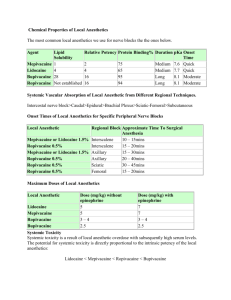
Avoiding systemic toxicity
• Use acceptable total dose
• Avoid intravascular administration (aspirate before injecting)
• Administer drug in divided doses
Maximum safe doses of local anesthetics in adults
Anesthetic
Procaine
Chloroprocaine
Tetracaine
Lidocaine
Mepivicaine
Bupivacaine
Dose (mg)
500
600
100 (topical)
300
300
175
Uses of Local Anesthetics
•Topical anesthesia
- Anesthesia of mucous membranes (ears, nose, mouth, genitourinary, bronchotrachial)
- Lidocaine, tetracaine, cocaine (ENT only)
•EMLA (eutectic mixture of local anesthetics) cream formed from lidocaine (2.5%) & prilocaine
(2.5%) penetrates skin to 5mm within 1 hr, permits superficial procedures, skin graft harvesting
•Infiltration Anesthesia w/o epinephrine)
- lidocaine, procaine, bupivacaine (with or
- block nerve at relatively small area
- anesthesia without immobilization or disruption of bodily functions
- use of epinephrine at end arteries (i.e.; fingers, toes) can cause severe vasoconstriction leading to gangrene
Uses of Local Anesthetics
•Nerve block anesthesia
- Inject anesthetic around plexus (e.g.; brachial plexus for shoulder and upper arm) to anesthetize a larger area
- Lidocaine, mepivacaine for blocks of 2 to 4 hrs, bupivacaine for longer
•Bier Block (intravenous)
- useful for arms, possible in legs
- Lidocaine is drug of choice, prilocaine can be used
- limb is exsanguinated with elastic bandage, infiltrated with anesthetic
- tourniquet restricts circulation
- done for less than 2 hrs due to ischemia, pain from touniquet
Uses of Local Anesthetics
•Spinal anesthesia
- Inject anesthetic into lower CSF (below L2)
- used mainly for lower abdomen, legs, “saddle block”
- Lidocaine (short procedures), bupivacaine
(intermediate to long), tetracaine (long procedures) for bowel surgery
- Rostral spread causes sympathetic block, desirable
- risk of respiratory depression, postural headache
Uses of Local Anesthetics
•Epidural anesthesia
- Inject anesthetic into epidural space
- Bupivacaine, lidocaine, etidocaine, chloroprocaine
- selective action of spinal nerve roots in area of injection
- selectively anesthetize sacral, lumbar, thoracic or cervical regions
- nerve affected can be determined by concentration
- High conc: sympathetic, somatic sensory, somatic motor
- Intermediate: somatic sensory, no motor block
- low conc: preganglionic sympathetic fibers
- used mainly for lower abdomen, legs, “saddle block”
- Lidocaine (short procedures), bupivacaine
(intermediate to long), tetracaine (long procedures) for bowel surgery
- Rostral spread causes sympathetic block, desirable