Leukemia
Leukemia is a general term for a
group of malignancies of either
lymphoid or hematopoietic cell origin.
General features:
1- The number of circulating leukocytes is often greatly
increased. In few cases the number is of normal range
(aleukemic leukemia).
2- The bone marrow is diffusely infiltrated with leukemic
cells, often with encroachment on normal
hematopoietic cell development.
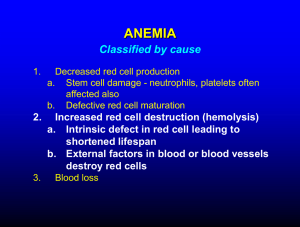
3- Consequent failure of normal leukocyte, red cell, and
platelet production which results in anemia, infection,
and hemorrhage.
3- Infiltration of leukemic cells in the liver, spleen, lymph
nodes, and other organs is common.
Pathological features of leukemia
1- Bone marrow:
-Shows leukoblastic reaction mainly in the sternum, ribs,
vertebrae, and pelvis.
- The leukemic tissue appears reddish brown in acute
leukemia and grayish-white in chronic leukemia.
2- Blood changes:
A- Increase of the total leucocytic count due to blood
invasion by the leukemic cells.
B- In acute leukemia the blast type of cells predominate,
while in chronic leukemia more differentiated cells
appear ( myelocytes, and lymphocytes).
C- Anemia especially in chronic leukemia.
D- Thromocytopenia.
3- Leukemic infiltrate:
- In the liver, adrenal, kidney…………and skin. The
infiltrate begins around the blood vessels and as it
increases in size it destroys the surrounding tissue.
4- Lymph nodes:
- The infiltrate causes enlargement of the nodes and loss
of the normal pattern. Affection of the perinodal tissue
fuses the nodes together.
5- Splenomegaly:
- Due to leukemic infiltrate and splenic infarcts caused by
thrombosis of its vessels.
Etiologic factors
The difficulty of pinpointing one cause of leukemias
indicates that it may be caused by a combination of
different factors:
1- Chromosomal translocations and oncogenesis: nonrandom karyotypic abnormalities, most commonly
translocations are present in the majority of leukemias.
2- Inhereted genetic factors: Down syndrome (trisomy
21) and neurofibromatosis are associated with increased
incidence of childhood leukemia.
3- Viruses:
Three viruses HTLV-1, EBV, and HHV-8 have been
implicated as causative agent of lympho-hemetopoietic
neoplasms
HTLV-1→ adult T-cell leukemia/lymphoma.
EBV
→ African Burkitt’s lymphoma.
HHV-8 → Primary effusion lymphoma.
4- Iatrogenic factors:
Radiotherapy and certain forms of chemotherapy used
to treat cancer increase the risk of subsequent leukemia
especially AML.
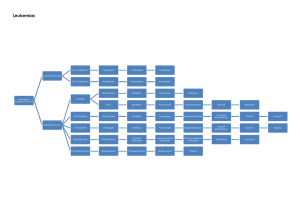
Classification:
- The classification of leukemia is based on:
1- The predominent cell type:
a- Myeloid leukemia.
b- Lymphatic leukemia.
c- Plasma cell and histiocytic leukemia (rare).
d- Monocytic leukemia.
2- The degree of cellular differentiation:
a- Acute leukemias (undifferentiated with rapid
course).
b- Chronic leukemias ( Moderately differentiated
with slow course.
Acute leukemias
General considerations:
1- A predominance of blasts and closely related cells in the bone
marrow and peripheral blood is characteristic.
2- The most common malignancies of the pediatric age group, acute
leukemias occur most often in children. They exhibit a second
incidence peak in the late adult age.
3- Cytogenetic abnormalities are frequent. For example, the 9;22
translocation results in a morphologically unique chromosome, the
Philadelphia chromosome (Ph 1). This abnormality, better known
for its association with chronic myelogenous leukemia, is associated
with a poorer prognosis when it occurs in acute leukemias.
4- Without therapeutic intervention, acute leukemia follows a short and
precipitous course, marked anemia, infection, and hemorrhage, and
death occurs within 6 to 12 months.
ACUTE LYMPHOBLASTIC LEUKEMIA (ALL)
EPIDEMIOLOGY: Childhood. Most common childhood malignancy.
PATHOGENESIS: The c-myc gene is involved, as in Burkitt
Lymphoma.
IMMUNOTYPES: Several different cell-types can yield ALL. Flow
Cytometry can be used to identify the specific type.
B-cell ALL: More common, better prognosis. Subtypes are Pre-PreB-ALL, Pre-BALL, and Mature B-ALL (worst prognosis)
T-Cell ALL: More common in adolescents, worst prognosis.
Null Cell ALL
SUBTYPES: 3 Subtypes proposed by the FrenchAmerican-British (FAB) Group:
• L1 LYMPHOBLASTS: Small, plain cells.
CLINICAL: Best prognosis, common in the children 3-7
age group.
• L2 LYMPHOBLASTS: Contain prominent nucleoli.
CLINICAL: Found in infants younger than 1, or common
in adolescents (T-cell immunotype) or in adults.
• L3 LYMPHOBLASTS: Burkitt's Leukemia. Identical
histology to Burkitt's Lymphoma. Larger cells with
vacuoles in cytoplasm, and showing characteristic
starry sky appearance .
CLINICAL: Poor prognosis, found in children 6-11 years of
age.
Clinically:
Hepatosplenomegaly
Generalized Lymphadenopathy, particularly
cervical nodes.
Normocytic normochromic anemia,
thrombocytopenia, neutropenia.
May have CNS involvement.
PATHOLOGY:
Numerous cases have cytogenetic abnormalities.
Hyper-diploidy is a common abnormality and is a
favorable prognostic indicator.
DIAGNOSTIC CRITERIA:
- Numerous blast cells must be present in the
bone marrow or peripheral blood.
- TDT -Positive: Terminal Deoxynucleotidyl
Transferase is present in the lymphoid cells,
distinguishing it from the myeloid cells (AML).
- Myeloperoxidase-negative: Only granulocytes
have myeloperoxidase.
- PAS-Positive: Lymphoblasts in general stain
positive for PAS.
Prognosis:
- In children, with intensive therapy the cure rate
is greater than 50% and may appear to be
approaches 75%.
PROGNOSTIC FACTORS:
Clinical
Features
Favorable
Prognosis
Unfavorable
Prognosis
FAB Subtype
L1
L2, and especially L3
Immunotype
B-Cell (Pre-Pre-B-AIl,
Pre-B-All )
• T-Cell ALL
• Mature B-Cell ALL
WBC Count at
Diagnosis
Less than 10,000
Greater than 50,000
Age
Children 3-7 (will
probably be L1
Children less than 1 (L2
subtype) subtype), or
older than 10 (L3
subtype)
Race
White
Black
Sex
Female
Male
Organ Involvement.
Minimal
Prominent
Hyperdiploidy
=
Cytogenetic
Abnormalities
Innumerable number of lymphoblasts (L1).
The WBC's seen here are lymphocytes, but they
are blasts--very immature cells with larger nuclei
that contain nucleoli. Such lymphocytes are
indicative of acute lymphoblastic leukemia (L2).
Burkitt’s leukemia (L3)
Acute
lymphocytic
leukemia (ALL)
Results in a highly
cellular marrow. The
marrow between the
pink bone trabeculae
seen here is nearly
100% cellular, and it
consists of leukemic
cells that have virtually
replaced or
suppressed normal
hematopoiesis.
Acute myeloid (myeloblastic) leukemia
(AML)
General concepts:
- AML occurs most often in adults.
- A predominance of myeloblasts and early promyelocytes is characteristic.
- AML responds to current therapy more poorly
than ALL.
- Further classification into several subgroups is
based on morphology, cytochemical
characteristics, surface markers, and genetic
alterations.
1. EPIDEMIOLOGY: Most common leukemia found in
adulthood.
2- PATHOGENESIS: Clonal disorder arising from an
aberrant myeloid precursor cell, which includes
myeloblasts, monoblasts, erythroblasts, and
megakaryoblasts.
Possible causes:
- Myelotoxic agents: Benzene, and chemotherapeutic
alkylating agents are most important ones.
- Radiation.
- Down Syndrome has propensity to lead to AML. Other
chromosomal abnormalities too.
- Myelodysplastic Disorders (Refractory Anemias) are
considered to be pre-leukemic.
3. PATHOLOGY:
• AUER RODS: rod-shaped crystalloids made of
primary granules characteristic of myeloblasts.
Found in AML but not ALL.
4. SUBTYPES: FAB divides it into 8 Subtypes:
MO, M1 - M7
M0: undifferentiated myeloblasts without
myeloperoxidase.
M1: undifferentiated myeloblasts with .
myeloperoxidase
M2: some promyelocytic differentiation, maybe a
(few Auer rods; * t(8;21) is distinctive.
M3: very granular promyelocytes, often
many Auer rods, DIC are distinctive.
M4: myeloid and monocytic differentiation
M5: monocytic differentiation only; * t(9;11)
M6: features of red cell precursors
predominate; "Di Guglielmo's
(erythroleukemia"
M7: platelet markers; acute marrow fibrosis
((* PDGF effect; reticulin).
DIAGNOSIS:
- Leukocytosis, with greater than 30 blasts are present in
the bone marrow or blood.
- TDT -Negative: Terminal Deoxynucleotidyl Transferase
is not present in myeloid cells, distinguishing it from
the lymphoid cells (ALL).
SYMPTOMS:
Granulocytopenia, anemia, thrombocytopenia. All the
myeloblasts encroach on normal bone marrow
function.
GRANULOCYTIC SARCOMA (CHLOROMA): Discrete
tumor masses infiltrated into soft tissues. Occurs
especially in bones around face and lymph nodes.
Stains positive (red) with Chloroacetate Esterase, which is
the diagnostic stain.
Here are very large, immature myeloblasts with many
nucleoli. A distincitve feature of these blasts is a linear
red "Auer rod" composed of crystallized granules. These
findings are typical for acute myelogenous leukemia
.(AML) that is most prevalent in young adults
Auer Rods
Leukemias typically fill up the marrow with abnormal cells,
displacing normal hematopoiesis. The marrow here is
essentially 100% cellular, but composed almost
exclusively of leukemic cells.
At high power, the bone marrow of a patient
with acute myelogenous leukemia is seen
here. There is one megakaryocyte at the
right center.
Left shift refers to •
presence of immature
white cells in the
peripheral blood, i.e.,
they're being mobilized
early from the bone
marrow. To tell an
extreme case
(WBC>up to 100,000
or so, i.e., a leukemoid
reaction, as in sepsis,
overwhelming TB, or
carcinomatosis) from
chronic granulocytic
leukemia
Leukemoid
reaction
chronic
granulocytic
leukemia
High level of LAP
Low level of LAP
Low absolute
basophil count
High absolute
basophil count
Absence of
Philadelphia
Philadelphia
chromosome (Ph')
chromosome (Ph') is present
Presence of toxic
granulation / toxic
vacuolization says
"infection",
Absence of toxic
granulation / toxic
vacuolization says
"leukemia".