Overview of hematologic malignancies
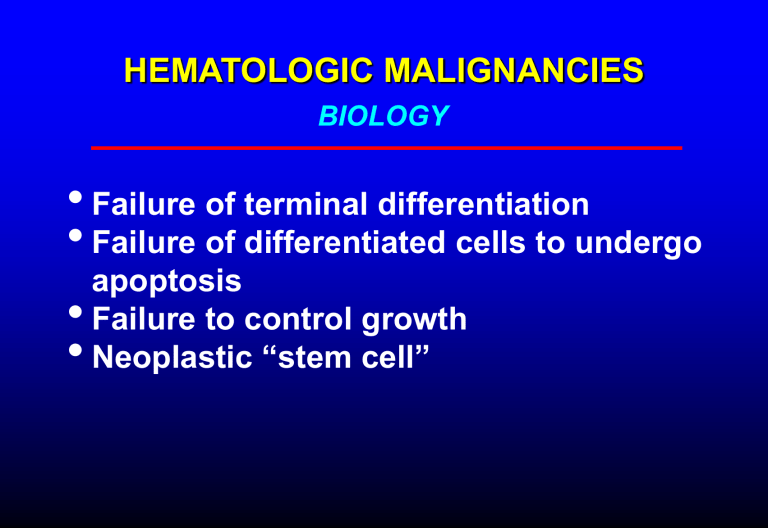
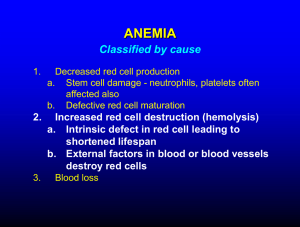
HEMATOLOGIC MALIGNANCIES
BIOLOGY
•
Failure of terminal differentiation
•
Failure of differentiated cells to undergo apoptosis
•
Failure to control growth
•
Neoplastic “stem cell”
FAILURE OF TERMINAL DIFFERENTIATION
•
Result: accumulation of rapidly dividing immature cells
•
Example: acute leukemias, aggressive lymphomas
FAILURE TO UNDERGO APOPTOSIS
•
Result: accumulation of relatively welldifferentiated, slow-growing cells
•
Example: chronic lymphocytic leukemia, indolent lymphomas
THE NEOPLASTIC STEM CELL
•
Propagation of malignant clone may depend on a subset of cells with stem cell-like properties
•
Some neoplastic stem cells retain the ability to differentiate into more than one cell type (eg, myeloproliferative/myelodysplastic disorders)
•
Eradication of neoplastic stem cell essential to cure disease?
•
Neoplastic stem cells may be slow-growing and resistant to treatment
Blood 2006;107:265
MYELOID NEOPLASIA
•
Myeloproliferative disorders
Polycythemia vera
Essential thrombocytosis
Myelofibrosis/myeloid metaplasia
Chronic myelogenous leukemia
•
Myelodysplasia
•
Acute myelogenous leukemia
MYELOPROLIFERATIVE DISORDERS
•
Affected cell: myeloid stem cell
All three cell lines affected; clonal hematopoiesis in most cases
•
Differentiation: normal to mildly abnormal
•
Kinetics: effective hematopoiesis
•
Marrow: hypercellular, variably increased reticulin fibrosis
•
Peripheral blood: increase in one or more cell lines in most cases
Exception: myelofibrosis
MYELOPROLIFERATIVE DISORDERS
•
•
Polycythemia Vera
•
Essential Thrombocythemia
Myelofibrosis/Myeloid Metaplasia
•
Chronic Myelogenous Leukemia
Polycythemia vera Essential thrombocythemia
Myeloid metaplasia CML
MARROW FIBROSIS
H&E Reticulin stain
MYELOPROLIFERATIVE DISORDERS
•
Diagnosis usually determined by peripheral blood counts
•
High Hct or platelet count may cause vaso-
• occlusive symptoms
•
Risk of portal vein thrombosis
Splenomegaly, constitutional symptoms frequent
•
Phlebotomy to control high Hct, hydroxyurea or other myelosuppressive Rx to control platelets, constitutional sx, etc
•
Transition to myelofibrosis or acute leukemia possible
VASO-OCCUSION IN POLYCYTHEMIA VERA
NEJM 2004; 350:99
NEJM 2004; 350:99
Mayo Clin Proc 2004;79:503
SPLENOMEGALY IN MYELOFIBROSIS
JAK2 MUTATION IN CHRONIC
MYELOPROLIFERATIVE DISORDERS
• Activation of JAK2 tyrosine kinase by cytokines initiates an important signaling pathway in myeloid cells
• A single point mutation of JAK2 (Val617Phe) has been identified in a high proportion (65-95%) of patients with polycythemia vera, and also in a substantial proportion of cases of essential thrombocytosis and myelofibrosis
• This mutation markedly increases the sensitivity of the cells to the effects of erythropoietin and other cytokine growth factors
• Testing for this mutation represents an important diagnostic tool
• This finding may lead to development new targeted therapies for myeloproliferative disorders
Mayo Clin Proc 2005;80:947
Diagnostic algorithm for polycythemia vera
Mayo Clin Proc 2005;80:947
CHRONIC MYELOGENOUS LEUKEMIA
BIOLOGY
•
Virtually all cases have t(9;22) (Ph1 chromosome) or variant translocation involving same genes
• bcr gene on chromosome 22 fused with abl gene on 9
•
Fusion gene encodes active tyrosine kinase
•
Clonal expansion of all myeloid cell lines
NEJM 2003;349:1451
NEJM 2003;349:1451
CHRONIC MYELOGENOUS LEUKEMIA
Blood smear Buffy coat Marrow biopsy
LEUKOSTASIS IN CML
NEJM 2005;353:1044
WBC 300K
CHRONIC MYELOGENOUS LEUKEMIA
Natural history
•
Incidence 1:100,000/yr
•
Peak incidence in 40s and 50s
•
Leukocytosis with mixture of mature and immature forms
•
Thrombocytosis common
•
Splenomegaly, constitutional symptoms, eventual leukostasis
•
Transition to acute leukemia (blast crisis) in 20%/yr
blasts may be myeloid or lymphoid essentially 100% mortality without BMT
CHRONIC MYELOGENOUS LEUKEMIA
TREATMENT
•
Gleevec (imatinib) – inhibits bcr-abl protein
• kinase
Hydroxyurea
•
Alfa interferon
•
Early allogeneic BMT in eligible pts (vs Gleevec
Rx?)
NEJM 2003;349:1399
MYELODYSPLASIA
•
Affected cell: myeloid stem cell
All cell lines affected, clonal hematopoiesis
•
Differentiation: mildly to severely abnormal
Morphology and function may be affected
•
Kinetics: Ineffective hematopoiesis (apoptosis of
• maturing cells in marrow)
•
Marrow: variable cellularity
Peripheral blood: decrease in one or more cell lines
(usually anemia with or without other cytopenias)
Platelets and WBC occasionally increased
•
Cytogenetic abnormalities frequent
•
Risk of transition to acute leukemia high when marrow blast count > 5%
MYELODYSPLASIA
WHO Classification
Myelodysplastic disorders
•
Refractory anemia
•
Refractory anemia with ringed sideroblasts
•
Refractory cytopenia with multilineage dysplasia
•
Refractory anemia with excess blasts-1 (5-10% blasts)
•
RAEB-2 (10-20% blasts)
Mixed myeloproliferative/myelodysplastic disorders
•
Chronic myelomonocytic leukemia
•
Atypical CML (bcr-abl negative)
SURVIVAL IN MYELODYSPLASIA
*
Overall survival Leukemia-free survival
*
Mortality of low-risk (RA) patients >70 no different from general population
J Clin Oncol 2005;23:7594
Myelodysplasia: blood smear
Myelodysplasia: blood smears with abnormal neutrophils
Myelodysplasia: marrows showing dyserythropoeisis and hypolobulated megakaryocyte
Myelodysplasia: acquired -thalassemia with Hgb H inclusions in
RBC. This is caused by somatic mutations in the -globin gene or an associated regulatory gene, limited to the neoplastic clone
Blood 2005;105:443
MDS: micromegakarycyte MDS: hypercellular marrow
MDS: ringed sideroblast CMML
RAEB – marrow blasts
RAEB – circulating blast, agranular PMN
MYELODYSPLASTIC SYNDROME
Myeloblast (red arrow) and abnl
RBC precursor (blue arrow)
ACUTE LEUKEMIA
Biology
• Leukemic clone: cells unable to terminally differentiate
– May be lymphoid or myeloid
– AML: May arise from abnormal stem cell
(eg in MDS/MPD) or de novo
• Accumulation of immature cells (blasts)
• Marrow replaced by leukemic cells
• Blasts accumulate in blood and other organs
ACUTE LEUKEMIA
Pathophysiology
•
Bone marrow failure
fatigue (anemia)
infection (neutropenia) bleeding (thrombocytopenia)
•
Tissue infiltration
organomegaly
skin lesions organ dysfunction pain
ACUTE LEUKEMIA
Pathophysiology (cont)
•
Leukostasis (WBC > 50-100K)
retinopathy
encephalopathy/CNS bleeding pneumonopathy
•
Biochemical effects of leukemic cell products
hyperuricemia/tumor lysis syndrome
DIC renal tubular dysfunction (lysozymuria)
lactic acidosis hypercalcemia (rare) spurious hypoglycemia/hypoxemia/hyperkalemia
Hyperleukocytosis in AML
NEJM 2003;349:767
Normal Patient
(WBC 250K)
26 yo with fever, encephalopathy, retinopathy, dyspnea, lymphadenopathy
ACUTE LEUKEMIA
Information used in classification
•
Clinical setting
•
Morphology
•
Histochemistry
•
Surface markers
•
Cytogenetics
•
Molecular genetics
ACUTE LEUKEMIA
Adverse prognostic features
•
•
Old age, poor performance status
•
Therapy-induced
Prior myelodysplastic/myeloproliferative
• disorder
•
High tumor burden
Cytogenetics: Ph 1 chromosome, deletion of 5 or
7, multiple cytogenetic abnormalities
ACUTE MYELOGENOUS LEUKEMIA
•
Affected cell: myeloid stem cell or committed progenitor cell
•
Differentiation: arrested at early stage, with absent or decreased maturation
•
Kinetics: marrow replacement by immature cells, decreased normal hematopoiesis
•
Marrow: usually markedly hyercellular with preponderance of blast forms
Hypocellular variants known
•
Peripheral blood: variable decrease in all cell lines with or without circulating immature cells
ACUTE MYELOGENOUS LEUKEMIA
Epidemiology
•
90% of adult acute leukemia: 2.2 deaths/100,000/yr
•
Incidence rises with age
•
Risk factors: exposure to ionizing radiation, alkylating agents and other mutagens (implicated in10-15% of all cases), certain organic solvents (benzene)
•
Precursor diseases: myelodysplastic & myeloproliferative disorders, myeloma, aplastic anemia, Down syndrome,
Klinefelter syndrome, Fanconi syndrome, Bloom syndrome
ACUTE MYELOGENOUS LEUKEMIAS
FAB (French-American-British) classification
•
M0 (minimal differentiation)
•
M1 (myeloid blasts)
•
M2 (some differentiation)
•
M3 (promyelocytic)
•
M4 (myelomonocytic)
•
M5 (monocytic)
•
•
M6 (erythroleukemia)
•
M7 (megakaryoblastic leukemia)
Unclassifiable (evolved from MDS, other secondary leukemias)
Newer classification schemes place more emphasis on cytogenetics and less on morphology
WHO classification of AML
• AML with recurrent cytogenetic abnormalities
– t(8;21)
– inv(16)
– Acute promyelocytic leukemia – t(15;17) and variants
– AML with 11q23 (MLL gene) abnormalities
• AML with multilineage dysplasia
•
AML/MDS, therapy-related
• AML not otherwise categorized
– Minimally differentiated
– Without maturation
– With maturation
– Acute myelomonocytic leukemia
– Acute monoblastic and monocytic leukemia
– Acute erythroid leukemia
– Acute megakaryblastic leukemia
– Acute basophilic leukemia
– Acute panmyelosis with myelofibrosis
– Myeloid sarcoma
• AML with ambiguous lineage
– Undifferentiated AML
– Bilineal AML
– Biphenotypic AML
ACUTE PROMYELOCYTIC LEUKEMIA
(APML; FAB M3)
• t (15;17)
•
Translocation involves retinoic acid
• receptor gene
•
High incidence of DIC/fibrinolysis
All-trans retinoic acid induces remission in high proportion of cases
•
Favorable prognosis
M0 M1
M2 M3
M4 M5
M6 M7
Auer rod in AML
ACUTE LYMPHOCYTIC LEUKEMIA
Classification
•
Morphology (FAB)
L1 (uniform)
L2 (pleomorphic)
L3 (Burkitt-type)
•
Immunophenotypic
B-cell (Burkitt-type, 2-3% of cases)
Pre-B cell (80% )
T-lineage
Mixed lineage (lymphoid-myeloid)
L1 ALL L2 ALL L3 ALL
ACUTE LYMPHOCYTIC LEUKEMIA
Epidemiology
•
About 3000 cases/yr in US
•
2/3 of cases in children (most common childhood cancer)
•
In adults, most cases in elderly
ACUTE LEUKEMIA
Treatment
•
Remission induction: aggressive combination chemotherapy
•
Post-remission
AML: consolidation (high-dose) or auto-BMT
ALL: consolidation, then maintenance (lower dose)
•
Allogeneic bone marrow transplant in selected patients
•
Cure rates 75%+ in childhood ALL; as high as 50% in "good risk" adults, up to 60% in BMT recipients
•
Overall cure rates still low in adults
SURVIVAL ACCORDING TO AGE IN PATIENTS WITH
FAVORABLE CYTOGENETICS TREATED FOR AML
(Excluding APML)
Blood 2006;107:3481
SURVIVAL ACCORDING TO AGE IN PATIENTS WITH
INTERMEDIATE CYTOGENETICS TREATED FOR AML
Blood 2006;107:3481
SURVIVAL ACCORDING TO AGE IN PATIENTS WITH
UNFAVORABLE CYTOGENETICS TREATED FOR AML
Blood 2006;107:3481
EFFECT OF AGE AND PERFORMANCE STATUS ON
EARLY MORTALITY IN TREATED AML
Blood 2006;107:3481