Febrile Neutropenia: A Review
advertisement
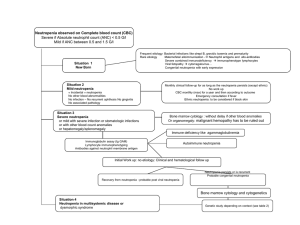
Febrile Neutropenia: A Review of the Guidelines September 29, 2010 Andrea Beaman, BScPhm, RPh PharmD Candidate Learning Objectives 1. Identify predisposing factors and common pathogens that cause infections in febrile neutropenic patients. 2. Review initial investigations that will help direct therapy in febrile neutropenic patients. Learning Objectives 3. Compare recommendations for empiric antibiotic selections for high risk and low risk febrile neutropenic patients. 4. Discuss assessment of response, treatment modifications and duration of therapy. Recent Guidelines National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prevention and Treatment of CancerRelated Infections v.2.2009 European Society for Medical Oncology. Management of Febrile Neutropenia: ESMO Clinical Practice Guidelines (2010) Infectious Disease Society of America. 2002 Guidelines for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer (Update expected Winter 2011) What is the Risk? Incidence of Febrile Neutropenia Induction-remission for AML 70-90% Elderly patients receiving CHOP 35-45% Patients with NHL 10-20% Mortality Estimates from Febrile Neutropenia Solid tumours 5% Hematological malignancy Up to 11% Gram-positive bacteremia 5% Gram-negative bacteremia 18% Case Presentation A 40 yr old woman diagnosed with locally advanced breast Ca in Mar/2010 Plan of care: Completed 3 cycles neoadjuvant FEC q3wk Received 2nd cycle Taxotere® q3wk on Sept 13 Receiving Neulasta® dose Day 1 postTaxotere® Plan for surgery in Nov. Case Presentation Presented on Sept 20th with Temp=39.5C and feeling “unwell”. WBC = 2.2 (4-11), Neutrophil = 0.42 (27.5) Creat = 87 (50-100) BP 170/60, RR 16 Ht = 178 cm, Wt = 90.7 kg Definition of Febrile Neutropenia Does this patient have febrile neutropenia? Fever: Single oral temperature ≥38.3°C or persistent temperature ≥38.0 °C for >1 hour. Temp 39.5 °C Neutropenia: ANC <0.5, or ANC <1.0 and a predicted decline to <0.5 over next 48 hrs. (ANC= absolute neutrophil count) ANC 0.42 Predisposing Factors Malignancy Type Advanced/refractory Obstructive Surgical risk Grade of neutropenia Disruption of mucosal barriers Corticosteroid use Microbiology Mainly gram-positive organisms (~70%) Coagulase-negative staphylococci S. aureus S.viridans Enterococci Gram-negative organisms Coliforms (E.coli, Klebsiella, Enterobacter) P.aeruginosa Yeast Candida Aspergillus Viruses Herpes simplex (HSV) Influenza, paranifluenza CMV Initial Investigations History & physical exam Lab assessments Diagnostic imaging Microbiologic evaluations Detailed H&P Including Chemotherapy regimen & last dose given Presence of vascular devices Prophylactic antibiotic Steroid use Allergies Major comorbid illnesses Recent surgical procedures Recent infections or positive cultures Previous antibioticresistant organisms or bacteraemia Recent exposures Site-Specific H&P Oropharynx Respiratory system GI tract Skin Genitourinary CNS No mucositis Mild cough No N/V/D No skin lesions, CVAD Yeast Infection No CNS symptoms Lab assessments CBC with differential BUN, SCr Electrolytes LFTs Urinalysis WBC=2.2, ANC=0.42, Hgb=99, Plt=345 BUN=3, SCr=87 Na=139, K=3.6, Cl=104, HCO3=28 Tbili=16, ALT=117, AST=76, Alp=84 Normal Microbiologic evaluations Blood cultures x2 1 catheter + 1 peripheral 2 catheter 2 peripheral Urine culture if symptomatic urinary catheter or abnormal urinalysis Blood & urine cultures Negative Site-Specific Cultures Diarrhea: C.difficile assay, stool microscopy and culture Sputum microscopy and culture Aspirate/swab/biopsy of any skin lesions or CVAD-associated symptoms Viral cultures Vesicular or ulcerated skin/mucosal lesions Throat or nasopharynx for respiratory symptoms (esp. during outbreaks) LP if CNS symptoms Fungal cultures Broad Spectrum Patient Assessment Organ Function AntiPseudomonal Local ABx Susceptibility Potential Organisms Site of Infection Initial Therapy Allergy Status Bactericidal Previous ABx Use Risk Status Assessment Low Risk High Risk Outpatient at time of fever Inpatient at time of fever No acute comorbid illnesses Significant medical comorbidity Anticipated short duration of severe neutropenia Anticipated severe or prolonged neutropenia No renal insufficiency CrCL <30 ml/min No hepatic insufficiency Transaminases ≥5x ULN Good performance status Uncontrolled/progressive cancer, Mucositis grade 3-4 MASCC Risk Index score ≥21 MASCC Risk Index score <21 LOW RISK Complex infection MASCC Index Multinational Association for Supportive Care in Cancer Prospectively validated tool to rapidly assess risk before access to neutrophil count. Scores 21 are at low risk of complications (max score 26). MASCC Score=26 MASCC scoring index: Burden of illness: no or mild symptoms 5 Burden of illness: moderate symptoms 3 Burden of illness: severe symptoms 0 No hypotension (systolic BP >90 mmHg) 5 No chronic obstructive pulmonary disease 4 Solid tumour/lymphoma with no previous fungal infection 4 No dehydration 3 Outpatient status at onset of fever 3 Age <60 years (not valid in children <18 years) 2 Klastersky J,J Clin Oncol 2000; 18:3038–51. Low Risk Treatment Low risk, adult patients No focus of infection, hemodynamically stable No systemic symptoms other than fever No organ failure, pneumonia, soft tissue infection, CVAD Recovering bone marrow Reliable patient Vigilant observation Access to medical care 24-7 Return to clinic if Positive cultures Persistent/recurrent fever @ 3-5 days Unable to tolerate PO regimen Cipro 500 mg PO Q8h + amoxicillin-clavulanate 500 mg PO Q8h Principles of High Risk Treatment Inpatient treatment with IV antibiotics Coverage for MRSA or resistant Gram- negative bacteria may be required. B-lactam antibiotic in combination with an aminoglycoside is preferable to monotherapy with antipseudomonal cephalosporins. IV Monotherapy Cefepime Imipenem-cilastin Meropenem** Piperacillin-tazobactam** (NCCN) Ceftazidime** (with concerns) **Formulary (all others Non-formulary at THC) IV Combination Therapy Advantages: Synergistic effect against gramnegatives Reduced emergence of resistance Disadvantages: Lack of activity against grampositives? Toxicity IV Combination Therapy Aminoglycoside + (meropenem, imipenem-cilastin or piperacillintazobactam) Aminoglycoside + (cefepime or ceftazidime) Ciprofloxacin + (meropenem, imipenem-cilastin or piperacillintazobactam) IV Therapy Options: Comparison Piperacillin-tazobactam Broad spectrum gram(-), gram(+) & anaerobic coverage Use for intra-abdominal source Not recommended for meningitis (poor CSF penetration) Imipenem-cilastin Broad spectrum gram(-), gram(+) & anaerobic and ESBL coverage Use for intra-abdominal source Risk of seizures in CNS malignancy or renal impairment IV Therapy Options: Comparison Meropenem Broad spectrum gram(-), gram(+) & anaerobic and ESBL coverage Use for intra-abdominal source Preferred for meningitis/CNS infection Ceftazidime Poor gram(+) activity Breakthrough streptococcal infections No activity against anaerobes, enterococcus Good CSF penetration IV Treatment Options: Comparison Aminoglycosides Gram(-) coverage, synergy with beta-lactams against S.aureus and Enterococcus Nephrotoxicity, ototoxicity Ciprofloxacin Gram(-) and atypical bacterial coverage No anaerobic coverage, less gram(+) activity than other options Good clinical studies as empirical PO or IV therapy Avoid in patients recently treated with quinolone prophylaxis Vancomycin Vancomycin not routinely recommended for empiric therapy Use should be limited to specific indications: clinically suspected serious catheter-related infection known colonization with MRSA or pcn/ceph-resistant pneumococci gram-positive bacteremia pending further C&S hypotension or other cardiovascular impairment soft-tissue infection risk factors for viridans strep bacteremia (severe mucositis + prophylaxis with Septra or Cipro) Reassess Vancomycin after 24-48 hours THC protocol: Ceftazidime 2g IV q8h Gentamicin 120 mg IV q12h x2 doses, then Pharmacist to dose Case Patient Gentamicin Dosing: ABW=90.7 kg, Ht = 178 cm IBW=70.7 kg, DW=78.8 kg SCr=73, CrCl=92 ml/min Gentamicin once daily dosing appropriate Dose: Age 16-65: 6 mg/kg (DW)/dose = 460 mg IV Frequency: CrCl >60 ml/min: Q24h Antifungals as Empiric Therapy? Clinical suspicion: High risk patients with prolonged neutropenia and site-specific symptoms: Oral thrush: Mucositis mouthwash , Fluconazole Esophageal lesions: Fluconazole Sinus/nasal symptoms and suspicious CT/MRI: Amphotericin B Pneumonia: voriconazole, amphotericin B Empiric treatment required based on H&P as positive cultures can take several days. Antifungals Added Later? IDSA recommends consider antifungal if febrile after 3-5 days and remains neutropenic Amphotericin B is preferred Fluconazole may be acceptable at institutions with low rates of mold infections or drug-resistant Candida species Antifungals Added Later? NCCN recommends: Add fluconazole if no prior azole antifungal prophylaxis, low risk for invasive aspergillosis and low rates of azole-resistant Candida. Dosing: 150 mg PO x1 dose for vaginal candidiasis 200 mg PO daily x14 days for candidal pyelonephritis 800 mg x1 then 400 mg daily x14 days from first negative culture for candidiasis (not recommended if received prophylaxis) 400 mg PO daily prophylaxis for neutropenic patients Antifungals Added Later? NCCN Recommends Add voriconazole, liposomal amphotericin B or an echinocandin if already exposed to an azole or known to be colonized with nonalbicans Candida. Voriconazole 6 mg/kg IV q12h x2 doses then 4 mg/kg IV/PO q12h Amphotericin B 3-5 mg/kg IV daily Caspofungin 70 mg IV x1 then 50 mg IV daily; 70 mg IV daily for aspergillosis Continue until neutropenia has resolved, or for at least 14 days in patients with a demonstrated fungal infection. Case Presentation Does she need antifungal coverage? Symptomatic vaginal yeast infection, unsuccessfully treated Fluconazole 200 mg PO x1 dose given. Clotrimazole Vaginal ovule daily x3 days with prn use of cream. When to Add Antiviral Therapy Oral vesicular lesions: HSV Esophageal lesions: HSV, CMV Skin lesions: VZV Pneumonia: Influenza CNS symptoms: HSV Antiviral Doses Acyclovir: Mucocutaneous HSV: 5 mg/kg IV Q8h Single dermatomal VZV: 800 mg PO 5x/day or 5 mg/kg IV Q8h Disseminated VZV or HSV: 10 mg/kg IV Q8h Valacyclovir: HSV or VZV treatment: 1g PO Q8h Ganciclovir: CMV treatment: 5 mg/kg IV Q12h x2 weeks then 5 mg/kg IV Q24h x2-4 weeks Foscarnet: Acyclovir-resistant HSV: 40 mg/kg IV Q8h CMV treatment: 90 mg/kg IV Q12h x2 weeks then 120 mg/kg IV Q24h x2-4 weeks Oseltamivir: Influenza: 75 mg PO Q12h (reduced doses required in renal impairment) Pneumonia Additional Tests: Include coverage for: sputum cultures atypical bacteria with Nasal wash for respiratory viruses Legionella urine antigen test Consider BAL If high risk consider adding CT chest to define infiltrates ID Consult azithromycin P.jirovecii with Septra MRSA with vancomycin or linezolid adding antiviral therapy (influenza outbreak) mold-active antifungal (voriconazole or liposomal amphotericin B) if high risk Gastrointestinal Symptoms Abdominal pain Abdominal CT or ultrasound ALP, transaminases, bilirubin, amylase, lipase Ensure anaerobic coverage Diarrhea C.difficile assay, (rotavirus & norovirus?) Consider stool bacterial cultures +/- parasite exam Metronidazole if C.difficile suspected Urinary tract symptoms Urine culture Urinalysis No additional therapy until pathogen identified CNS Symptoms ID consult Neurology consult CT +/- MRI LP recommended Empiric therapy: Anti-pseudomonal penicillin that enters CSF (ceftazidime, meropenem) Vancomycin Ampicillin unless using meropenem For encephalitis add high dose acyclovir Assessment of Response Daily assessment until afebrile and ANC 0.5: Fever CBC Renal function Clinical Symptoms Case Presentation Assessment of Response Sept 21 Sept 22 Sept 23 WBC 3.6 ANC 0.9 SCr=75 CrCl 92 ml/min Temp=37 WBC 4.0 ANC 1.4 SCr=73 WBC 5.4 ANC 3.7 Temp=37.4 Temp=37.7 Urine C&S negative, CXR clear Gent trough 0.4 Decision to discharge in 24hr. Duration of Therapy Afebrile and ANC 0.5 x48 hrs: Low risk patients, no source of infection identified: can discontinue abx High risk patients or with documented infection: continue tailored therapy 7 days Afebrile but ANC <0.5 after 5-7 days: low risk: can discontinue abx high risk: continue abx until ANC 0.5 or 14 days in pts not expecting ANC recovery. Febrile: Neutropenic: continue abx at least 14 days, reassess for non-response Non-neutropenic: discontinue abx 4-5 days after ANC >0.5 if no source of infection identified IDSA 2002 Guidelines for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer Follow up for Non-Responsive Patients Febrile but otherwise stable If non-neutropenic consider d/c abx 4-5 days after ANC >0.5 Consider antifungal therapy with activity against mold if fever continuing ≥4-5 days. Febrile and clinically unstable Broaden coverage to include anaerobes, resistant gram negative rods, resistant gram positive organisms Ensure coverage of Candida Consider antifungal therapy with activity against mold if fever continuing ≥4 days of therapy ID consult Duration of Therapy for Documented Infection Skin/soft tissue: 7-14 days Sinusitis: 10-21 days Bacterial pneumonia: 10-21 days Duration of Therapy for Documented Infection Uncomplicated bacteremia: Gram negative: 10-14 days Gram positive: 7-14 days S.aureus: at least 2 weeks after first negative blood culture and normal TEE Yeast: ≥2 weeks after first negative blood culture Duration of Therapy for Documented Infection mold (aspergillus etc): min 12 weeks Viral: HSV/VZV: 7-10 days Influenza: ≥5 days. Where do we go from here? The role of oral therapy and IV monotherapy? Antibiotic lock solutions for CVADs The role of G-CSFs Updated IDSA Guidelines Questions??