Chapter 19
The Peripheral Endocrine Glands
Human Physiology by Lauralee Sherwood ©2007 Brooks/Cole-Thomson Learning
Peripheral Endocrine Glands
• Outline
• Thyroid glands
– Anatomy and hormones
• Adrenal glands
– Anatomy and hormones
– Stress response
• Fuel metabolism
• Calcium metabolism
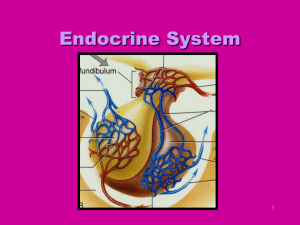
Thyroid Gland
• Consists of two lobes of endocrine tissue joined in middle by
narrow portion of gland
• Follicular cells
– Arranged into hollow spheres
– Forms functional unit called a follicle
– Lumen filled with colloid
• Serves as extracellular storage site for thyroid hormone
– Produce two iodine-containing hormones derived from
amino acid tyrosine
• Tetraiodothyronine (T4 or thyroxine)
• Tri-iodothyronine (T3)
• C cells
– Secrete peptide hormone calcitonin
Thyroid Gland
Fig. 19-1b, p. 684
Fig. 19-2, p. 685
Thyroid hormone synthesis
•
•
•
•
•
Tg = thyroglobulin
Transport of Tg to colloid
Iodine uptake to colloid
Iodination of tyrosine
coupling
Thyroid hormone release
• Stored as Tg
• Phagocytes “bite” Tg containing colloid
• Phagocytes cleave T3 and T4 from Tg in the
follicular cells
• T3 and T4 diffuse to blood (TBG carrier protein in
blood)
• Iodine is recycled after metabolism
• Activity
– 90% T4 but T3 4X aspotent
Colloid
Blood
Thyroid follicular cell
*Endoplasmic
reticulum/Golgi
complex
Lysosome
TGB = Thyroglobulin
I = Iodine
MIT = Monoiodotyrosine
DIT = Di-iodotyrosine
T3 = Tri-iodothyronine
T4 = Tetraiodothyronine (thyroxine)
Fig. 19-2, p. 685
Thyroid Gland
• Effects of thyroid hormone
– Main determinant of basal metabolic rate and
heat production
– Influences synthesis and degradation of
carbohydrate, fat, and protein (intermediary
metabolism)
– Increases target-cell responsiveness to
catecholamines (sympathomimetic effect)
– Increases heart rate and force of contraction
– Essential for normal growth
– Plays crucial role in normal development of
skeleton and nervous system (stimulates GH and
IGF-1)
Thyroid Gland
• Secretion
– Regulated by negative-feedback system between
hypothalamic TRH, anterior pituitary TSH, and
thyroid gland T3 and T4
– Feedback loop maintains thyroid hormones
relatively constant
Stress
Cold in
infants
Hypothalamus
Thyrotropinreleasing
hormone (TRH)
Anterior pituitary
Thyroid-stimulating
hormone (TSH)
Thyroid gland
Thyroid hormone
(T3 and T4)
Metabolic rate and heat production;
enhancement of growth and CNS
development; enhancement of
sympathetic activity
Fig. 19-3, p. 687
Thyroid Gland Dysfunction
Table 19-1, p. 687
Thyroid Gland
• Abnormalities
– Hyperthyroidism
• Most common cause is Graves’ disease
– Autoimmune disease
– Body erroneously produces thyroid-stimulating
immunoglobulins (TSI)
– Characterized by exopthalmos
• Treatment
– Surgical removal of a portion of the over-secreting thyroid
– Administration of radioactive iodine
– Use of antithyroid drugs
Graves disease
Antibody that binds
TSH receptors
no negative FB
Fig. 19-4, p. 688
Hyperthyroidism - exopthalmos
Fig. 19-5, p. 688
Thyroid Gland
• Abnormalities
– Hypothyroidism
• Causes
– Primary failure of thyroid gland
– Secondary to a deficiency of TRH, TSH, or both
– Inadequate dietary supply of iodine
• Cretinism
– Results from hypothyroidism from birth
• Myxedema
– Term often used for myxedema in adults
• Treatment
– Replacement therapy
– Dietary iodine
Hypothyroidism
Goiter
Fig. 19-6, p. 689
Adrenal Glands
• Embedded above each kidney in a capsule of fat
• Composed of two endocrine organs
– Adrenal cortex
• Outer portion
• Secretes steroid hormones
– Adrenal medulla
• Inner portion
• Secretes catecholamines
Adrenal
cortex
Anatomy
Adrenal
medulla
Zona glomerulosa
Cortex
Zona fasciculata
(See next slide)
Zona reticularis
Medulla
Adrenal gland
(C) Brooks/Cole - Thomson Learning
Fig. 19-7, p. 690
Adrenal Glands
Zona glomerulosa
Zona fasciculata
(See next slide)
Zona reticularis
• Adrenal cortex
– Consists of three layers or zones
• Zona glomerulosa – outermost layer
(Mineralocorticoids)
Medulla
• Zona fasciculata – middle and largest portion
(Glucocorticoids)
• Zona reticularis – innermost zone (Sex hormones)
– Categories of adrenal steroids
• Mineralocorticoids
– Mainly aldosterone
– Influence mineral balance, specifically Na+ and K+
balance
• Glucocorticoids
– Primarily cortisol
– Major role in glucose metabolism as well as in protein and
lipid metabolism
• Sex hormones
– Identical or similar to those produced by gonads
– Most abundant and physiologically important is
dehydroepiandosterone (male “sex” hormone)
Connective tissue
capsule
Zona
glomerulosa
Zona
fasciculata
Cortex
Zona
reticularis
Medulla
Fig. 19-7, p. 690
Cholesterol
Pregnenolone
Progesterone
Zona glomerulosa
Corticosterone
Aldosterone
Cholesterol
Pregnenolone
17-OH-Pregnenolone
Zona fasciculata
17-OH-Progesterone
Cortisol
Cholesterol
Pregnenolone
Zona reticularis
17-OH-Pregnenolone
fig 14-5, pg 437
Dehydroepiandrosterone
Effects of Adrenal Cortical Hormones
• Permissive actions on catecholamines
• Stress adaptation (releases building blocks of new
tissue)
• Anti-inflamatory and immunosuppressive
Regulation of cortisol
• ACTH from POMC
• Negative feedback
Diurnal
rhythm
Stress
Hypothalamus
Corticotropin-releasing
hormone (CRH)
Anterior pituitary
Adrenocorticotropic
hormone (ACTH)
Adrenal cortex
Cortisol
Metabolic fuels
and building blocks
available to help
resist stress
Blood glucose
(by stimulating gluconeogenesis
and inhibiting glucose uptake)
Blood amino acids
(by stimulating protein degradation)
Blood fatty acids
(by stimulating lipolysis)
Fig. 19-8, p. 692
Adrenal Glands
• Cortisol
• Zona fasciculata – middle and largest portion (Glucocorticoids)
– Stimulates hepatic gluconeogenesis
– Inhibits glucose uptake and use by many tissues, but not the
brain
– Stimulates protein degradation in many tissues, especially
muscle
– Facilitates lipolysis
– Plays key role in adaptation to stress
– At pharmacological levels, can have anti-inflammatory and
immunosuppressive effects
• Long-term use can result in unwanted side effects
– Displays a characteristic diurnal rhythm (day hi night low)
– Secretion
• Regulated by negative-feedback loop involving hypothalamic CRH
and pituitary ACTH
Adrenal Glands
• Secretes both male and female sex hormones in
both sexes
– Dehydroepiandrosterone (DHEA)
• Zona reticularis – innermost zone (Sex hormones)
• Only adrenal sex hormone that has any biological
importance
• Overpowered by testicular testosterone in males
• Physiologically significant in females where it governs
– Growth of pubic and axillary hair
– Enhancement of pubertal growth spurt
– Development and maintenance of female sex drive
Hypothalamus
GnRH
CRH
Anterior pituitary
FSH, LH
ACTH
Gonads
Adrenal cortex
Enzyme
absent
No sex hormone production No gamete
(androgens or estrogens)
production
Androgen
No
cortisol
Virilization
= Normal pathway that does not occur
FSH = Follicle-stimulating hormone
ACTH = Adrenocorticotropic hormone
LH = Luteinizing hormone
GnRH = Gonadotropin-releasing hormone CRH = Corticotropin-releasing hormone
Fig. 19-10, p. 695
Adrenal Glands
• Aldosterone
• Zona glomerulosa – outermost layer
(Mineralocorticoids)
– Secretion is increased by
• Activation of renin-angiotensin-aldosterone system by
factors related to a reduction in Na+ and a fall in blood
pressure
• Direct stimulation of adrenal cortex by rise in plasma K+
concentration
– Regulation of aldosterone secretion is largely
independent of anterior pituitary control
Disorders of Adrenocortical Function
• Aldosterone hypersecretion
– Primary hyperaldosteronism or Conn’s syndrome
• Cortisol hypersecretion
– Cushings syndrome
• Adrenal androgen hypersecretion
– Hirutism, female pseudohermaphrodism,
precosious pseudopuberty
• Primary adrenocortical insuficiency
– Addison’s disease
Disorders of Adrenocortical Function
• Aldosterone hypersecretion
– May be caused by
• Hypersecreting adrenal tumor made up of aldosteronesecreting cells
– Primary hyperaldosteronism or Conn’s syndrome
• Inappropriately high activity of the renin-angiotensin
system
– Secondary hyperaldosteronism
– Symptoms
• Excessive Na+ retention and K+ depletion
• High blood pressure
Disorders of Adrenocortical Function
• Cortisol hypersecretion
– Cushing’s syndrome
– Causes
• Overstimulation of adrenal cortex by excessive
amounts of CRH and ACTH
• Adrenal tumors that uncontrollably secrete cortisol
independent of ACTH
• ACTH-secreting tumors located in places other than the
pituitary
– Signs and symptoms
• Hyperglycemia and glucosuria (adrenal diabetes)
• Abnormal fat distributions
– “buffalo hump” and “moon face”
Fig. 19-9, p. 694
Disorders of Adrenocortical Function
• Adrenal androgen hypersecretion
– Adrenogenital syndrome
– Symptoms
• Adult females
– Hirsutism
– Deepening of voice, more muscular arms and legs
– Breasts become smaller and menstruation may cease
• Newborn females
– Have male-type external genitalia
• Prepubertal males
– Precocious pseudopuberty
• Adult males
– Has no apparent effect
Disorders of Adrenocortical Function
• Adrenocortical insufficiency
– Primary adrenocortical insufficiency
• Addison’s disease
• Autoimmune disease
– Aldosterone deficiency
» Hyperkalemia and hyponatremia
– Cortisol deficiency
» Poor response to stress
» Hypoglycemia
» Lack of permissive action for many metabolic activities
– Secondary adrenocortical insufficiency
• Occurs because of pituitary or hypothalamic abnormality
• Only cortisol is deficient
Adrenal Medulla
• Modified part of sympathetic nervous system
• Primary stimulus for increased adrenomedullary secretion
activation of sympathetic nervous system by stress
• Releases epinephrine and norepinephrine
– Secreted into blood by exocytosis of chromaffin granules
– Vary in their affinities for the different adrenergic receptor
types
• Epinephrine
– Reinforces sympathetic system in mounting general
systemic “fight-or-flight” responses
– Maintenance of arterial blood pressure
– Increases blood glucose and blood fatty acids
Table 19-2, p. 697
CNS
Receptor type
target cells
PNS
Somatic
Preganglionic
Postaganglionic
Autonomic sympathetic
Autonomic sympathetic
Autonomic paraysmpathetic
Brainstem
Autonomic parasympathetic
Nicotinic
Alpha-receptors
Beta receptors
Muscarinic
Norephinephrine
Epinephrine
Acetylcholine
Nicotinic
Somatic alpha-motor neuron
Adrenal gland
Skeletal muscle
NE (15%)
Thoracic
E (85%)
Autonomic
sympathetic
Alpha
Blood stream
Chromaffin cell
Sympathetic ANS
Beta
Autonomic
sympathetic
Sacral
Ganglia
Muscarinic
Autonomic parasympathetic
Parasympathetic ANS
fig 10-6, pg 343
Stress Response
• Pattern of reactions to a situation that threatens
homeostasis
• Stress
– Generalized nonspecific response of body to any
factor that overwhelms or threatens to overwhelm
the body’s ability to maintain homeostasis
• Stressor
– Any noxious stimulus that brings about the stress
response
Shivering, fever, inflammation, etc.
General adaptation syndrome
Fig. 19-11, p. 698
General Adaptation Syndrome
• Alarm reaction- fight or flight response,muscles
tense, HR and BP increase
• Resistance or adaptation-nervous and endocrine
systems deal with stressor. dangerous if long term.
• Exhaustion-resistance drops, immunity suppression,
depletion of energy reserves, stress related disease.
Stress Response
• All the actions are coordinated by the hypothalamus
• Generalized stress response
– Activation of sympathetic nervous system
accompanied by epinephrine secretion
• Prepares body for fight-or-flight response
– Activation of CRH-ACTH-cortisol system
• Helps body cope by mobilizing metabolic resources
– Elevation of blood glucose and fatty acids
• Decreased insulin and increased glucagon secretion
– Maintenance of blood volume and blood pressure
• Increased activity of renin-angiotensin-aldosterone
system and increased vasopressin secretion
Stressor
Hypothalamus
CRH
Sympathetic
nervous
system
Posterior
pituitary
Anterior
pituitary
ACTH
Vasopressin
Adrenal medulla
Adrenal cortex
Epinephrine
Cortisol
Glucagon-secreting cells
Insulin-secreting cells
Arteriolar
Endocrine
pancreas
smooth muscle
Vasoconstriction
Glucagon
Insulin
Blood flow
through kidneys
Renin
Angiotensin
Aldosterone
Fig. 19-12, p. 700
Table 19-3, p. 699
Endocrine Control of Fuel Metabolism
• Metabolism
– All the chemical reactions that occur within the cells of the
body
• Intermediary metabolism or fuel metabolism
– Includes reactions involving the degradation, synthesis,
and transformation of proteins, carbohydrates, and fats
• Nutrient molecules are broken down through the process of
digestion into smaller absorbable molecules
– Proteins → amino acids
– Carbohydrates → monosaccharides (mainly glucose)
– Dietary fats (triglycerides) → monoglycerides and free fatty
acids
Table 19-4, p. 701
Anabolism and Catabolism
• Anabolism
– Buildup or synthesis of larger organic macromolecules
from small organic subunits
– Reactions usually require ATP energy
– Reactions result in
• Manufacture of materials needed by the cell
• Storage of excess ingested nutrients not immediately needed
for energy production or needed as cellular building blocks
• Catabolism
– Breakdown or degradation of large, energy-rich organic
molecules within cells
– Two levels of breakdown
• Hydrolysis of large cellular molecules into smaller subunits
• Oxidation of smaller subunits to yield energy for ATP
production
Food intake
Dietary protein
Dietary
carbohydrate
Dietary triglyceride
fat
D I G E S T I O N
Absorbable units
Amino
acids
Glucose
Fatty
acids
Monoglycerides
A B S O R P T I O N
Metabolic pool
in body
Body proteins
(structural or
secretory
products)
Storage, structural, and
functional
macromolecules in cells
Amino
acids
Glycogen storage
in liver and
muscle
Glucose
Triglycerides
in adipose tissue
stores (fat)
Fatty
acids
Urea
Urinary excretion
(elimination from body)
Oxidation to
CO2 + H2O + ATP (energy)
Expired
(elimination from body)
Use as metabolic fuel
in cells
Fig. 19-13, p. 702
Interconversions Among Organic Molecules
• Most interconversion of organic molecules occurs in liver
• Essential nutrients (certain amino acids and vitamins)
• Food intake is intermittent – nutrients must be stored for use
between meals
– Excess circulating glucose
• Stored in liver and muscle as glycogen
• Once liver and muscle stores are “filled up”, additional
glucose is transformed into fatty acids and glycerol and
stored in adipose tissue
– Excess circulating fatty acids
• Become incorporated into triglycerides
– Excess circulating amino acids
• Converted to glucose and fatty acids
Stored Metabolic Fuel in the Body
Metabolic States
• Absorptive state
– Fed state
– Glucose is plentiful and
serves as major energy
source
• Postabsorptive state
– Fasting state
– Endogenous energy
stores are mobilized to
provide energy
Roles of Key Tissues in Metabolic States
• Liver
– Primary role in maintaining normal blood glucose levels
– Principal site for metabolic interconversions such as
gluconeogenesis
• Adipose tissue
– Primary energy storage site
– Important in regulating fatty acid levels in the blood
• Muscle
– Primary site of amino acid storage
– Major energy user
• Brain
– Normally can only use glucose as an energy source
– Does not store glycogen
• Mandatory blood glucose levels be maintained
Pancreas
Alpha cell
Beta cell
Delta cell
Capillaries
Fig 15-3, pg 455
Pancreatic Hormones
• Pancreas
– Endocrine cells – Islets of Langerhans
• Β (beta) cells
– Site of insulin synthesis and secretion
• Α (alpha) cells
– Produce glucagon
• D (delta) cells
– Pancreatic site of somatostatin synthesis
• PP cells
– Least common islet cells
– Secrete pancreatic polypeptide
– The function of PP is to self regulate the pancreas secretion
activities (endocrine and exocrine).
• Insulin and glucagon
– Most important in regulating fuel metabolism
Pancreatic Hormones
• Somatostatin
– Released from pancreatic D cells in direct
response to increase in blood sugar and blood
amino acids during absorption of a meal
– Prevents excessive plasma levels of nutrients
– Local presence of somatostatin decreases
secretion of insulin, glucagon, and somatostatin
itself
– Physiologic importance has not been determined
–
somatomedin
•
A peptide hormone (4 kD) that is produced in the liver and is released in response to growth hormone.
Somatomedin stimulates the growth of bone and muscle.
Pancreatic Hormones
• Insulin
– Anabolic hormone
– Promotes cellular uptake of glucose, fatty acids,
and amino acids and enhances their conversion
into glycogen, triglycerides, and proteins,
respectively
• Lowers blood concentration of these small organic
molecules
– Secretion is increased during absorptive state
• Primary stimulus for secretion is increase in blood
glucose concentration
Diabetes Mellitus
• Most common of all endocrine disorders
• Prominent feature is elevated blood glucose levels
– Urine acquires sweetness from excess blood
glucose that spills into urine
• Two major types
– Type I diabetes
• Characterized by lack of insulin secretion
– Type II diabetes
• Characterized by normal or even increased insulin
secretion but reduced sensitivity of insulin’s target cells
Comparison of Type I and Type II Diabetes
Acute Effects
of
Diabetes Mellitus
Pancreatic Hormones
• Glucagon
– Mobilizes energy-rich molecules from storage
sites during postabsorptive state
– Secreted in response to a direct effect of a fall in
blood glucose on pancreatic α cells
– Generally opposes actions of insulin
– No known clinical abnormalities caused by
glucagon deficiency or excess
• Excess of glucose can aggravate hyperglycemia of
diabetes mellitus
Endocrine Control of Calcium Metabolism
• Plasma Ca2+ must be closely regulated to prevent changes in
neuromuscular excitability
– Also plays vital role in a number of essential activities
•
•
•
•
•
Excitation-contraction coupling in cardiac and smooth muscle
Stimulus-secretion coupling
Maintenance of tight junctions between cells
Clotting of blood
Neurotransmitter release
– Hypercalcemia
• Reduces excitability
– Hypocalcemia
• Brings about overexcitability of nerves and muscles
• Severe overexcitability can cause fatal spastic contractions of
respiratory muscles
Endocrine Control of Calcium Metabolism
• Three hormones regulate plasma concentration of
Ca2+ (and PO43-)
– Parathyroid hormone (PTH)
– Calcitonin
– Vitamin D
Endocrine Control of Calcium Metabolism
• Calcitonin
– Hormone produced by C cells of thyroid gland
– Negative-feedback fashion
• Secreted in response to increase in plasma Ca2+
concentration
– Acts to lower plasma Ca2+ levels by inhibiting
activity of bone osteoclasts
– Unimportant except during hypercalcemia
Endocrine Control of Calcium Metabolism
• Parathyroid hormone (PTH)
– Secreted by parathyroid glands
– Primary regulator of Ca2+
• Raises free plasma Ca2+ levels by its effects on bone
kidneys, and intestines
– Essential for life
• Prevents fatal consequences of hypocalcemia
– Facilitates activation of Vitamin D
Endocrine Control of Calcium Metabolism
• Vitamin D
– Stimulates Ca2+ and PO43- absorption from
intestine
– Can be synthesized from cholesterol derivative
when exposed to sunlight
• Often inadequate source
– Amount supplemented by dietary intake
– Must be activated first by liver and then by
kidneys before it can exert its effect on intestines
Calcium Disorders
• PTH hypersecretion (hyperparathyroidism)
– Characterized by hypercalcemia and
hypophosphatemia
• PTH hyposecretion (hypoparathyroidism)
– Characterized by hypocalcemia and
hyperphosphatemia
• Vitamin D deficiency
– Children – rickets
– Adults – osteomalacia
Precursor in skin
(7-dehydrocholesterol)
Dietary vitamin D
Sunlight
Vitamin D3
Hydroxyl group (OH)
Liver enzymes
25-OH D3
PTH
Hydroxyl group
+
Plasma Ca2+
Kidney enzymes
Plasma PO4
3-
1, 25-(OH)2 D3
(active vitamin D)
Promotes intestinal
absorption
of Ca2+ and PO4 3-
Fig. 19-23, p. 723
Credit: © Mediscan/Visuals Unlimited
Rickets is a condition caused by a deficiency of vitamin D, especially in infancy and
childhood, with disturbance of normal ossification. The disease is marked by bending and
distortion of the bones under muscular action, by the formation of nodular enlargements on
the ends and sides of the bones, by delayed closure of the fontanels, pain in the muscles,
and sweating of the head. Vitamin D and sunlight together with an adequate diet are curative,
provided that the parathyroid glands are functional.
206184
Negative-feedback Loops Controlling Parathyroid
Hormone (PTH) and Calcitonin Secretion
Bone remodeling
• Bone deposition
• Bone resorption
• Osteoblasts – secrete matrix for calcium phosphate
– Stromal cells
• Osteocytes – retired osteoblasts
• Osteoclasts – resorb bone
– macrophages
– Acids breakdown calcium phosphate and matrix
– RANKL – accelerates osteoclast activity
– OPG – suppresses osteoclast activity and
development
Fig. 19-19, p. 718
Osteoporosis
• Bone thinning
• Increased osteoclast activity
• Reduced osteoblast activity
Osteoporosis from an 89 year-old female. SEM.
Endocrine Control of Calcium Metabolism
• Vitamin D
– Stimulates Ca2+ and PO43- absorption from
intestine
– Can be synthesized from cholesterol derivative
when exposed to sunlight
• Often inadequate source
– Amount supplemented by dietary intake
– Must be activated first by liver and then by
kidneys before it can exert its effect on intestines
Blood glucose
Blood glucose
cell
cell
cell
cell
Glucagon
Insulin
Glucagon
Insulin
Blood glucose
to normal
Blood glucose
to normal
Fig. 19-17, p. 713
Table 19-7b, p. 715
Table 19-8, p. 717
Central
canal
Osteocyte
Lamella
Canaliculi
Osteon
Blood vessel
from marrow
Central
canal
Vessel in central canal
Stepped art
Fig. 19-20, p. 719
Fig. 19-21a, p. 722
Osteocyte
Osteoblast
Osteocytic–
osteoblastic bone
membrane
Osteoblast
Mineralized
bone
Outer
surface
Blood vessel
Central canal
Bone fluid
Canaliculi
Lamellae
Fig. 19-21a, p. 722
Fig. 19-21b, p. 722
Relieves
Plasma PO43-
(Because of inverse relationship
between plasma PO43- and Ca2+
concentrations caused by solubility
characteristics of calcium phosphate
salt)
Plasma Ca2+
Kidneys
Parathyroid glands
Activated vitamin D
PTH
PO43- reabsorption
by kidneys
Ca2+ reabsorption
by kidneys
Urinary excretion
of Ca2+
Ca2+ absorption
in intestine
(Counteract each other)
Urinary excretion
of PO43-
No change in plasma Ca2+
PO43- absorption
in intestine
Plasma PO43Fig. 19-25, p. 725