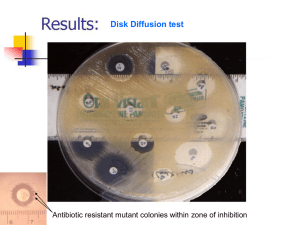
Culture - Pathology
advertisement

HAEMOPHILUS BORDETELLA Haemophilus sp. Organism Reservoir Transmission H. influenzae Normal flora of human upper resp. tract Person-to-person, droplets; sometimes endogenous H. ducreyi Not normal flora; only present during infection Person-to-person; sexual contact Other Haemophilus spp. Normal flora of human upper resp. tract Spread of endogenous strain to non-resp. tract sites; less common than H. influenzae Clinical characteristics H. influenzae Major virulence factor is polyribitol phosphate capsule - enhance resistance to phagocytosis - serologic typing based on antigenic characteristics - six capsule types: a, b, c, d, e, or f - type b is the most commonly associated with serious human infection - infections are often systemic and life-threatening: meningitis, epiglottitis, cellulitis with bacteremia, septic arthritis, and pneumonia Also produce factors that promote attachment to human epithelial cells Clinical characteristics, cont. H. influenzae Non-typeable strains do not produce a capsule - virulence mediated through attachment (pili, etc.) - infections are typically less serious and more localized: otitis media, sinusitis, conjuctivitis, and bronchitis - pneumonia and bacteremia in adults with underlying medical conditions - isolated from patients with cystic fibrosis Clinical characteristics, cont. H. ducreyi Virulence factors also uncertain but probably include capsule, pili and toxins involved in attachment and penetration human epithelial cells Etiologic agent of chanchroid - genital lesions beginning as tender papules that progress to painful ulcers with several satellite lesions - regional lymphadenitis - primarily occurs in lower socioeconomic groups in tropical areas Clinical characteristics, cont. H. ducreyi Chanchroid, cont. - can be distinguished from syphilitic lesions that are painless - presence of genital ulcers increases risk of HIV infection Clinical characteristics, cont. Other Haemophilus spp. Mainly low virulence, opportunistic pathogens Cause infections similar to H. influenzae but much less common H. aphrophilus is an uncommon cause of brain abscesses and endocarditis - H of HACEK; subacute bacterial endocarditis Laboratory Diagnosis Specimen collection • Can be isolated from most clinical specimens - relatively high bacterial load in blood of children with bacteremia • Susceptible to drying and temperature extremes • For H. ducreyi, specimen should be plated within 10 min. of collection Laboratory Diagnosis, cont. Direct detection • Gram stain: most are small, faintly staining, gram-negative coccobacilli H. ducreyi often described as “school of fish” - mostly seen in lymph node specimens http://www2.mf.uni-lj.si/~mil/bakt2/bakt2.htm Laboratory Diagnosis, cont. Direct detection • Latex agglutination can be performed on CSF or urine - can be falsely-positive for recent vaccinees - sensitivity is equivalent to Gram stain Laboratory Diagnosis, cont. Culture • Haemophilus require hemin (X factor) and NAD (V factor) Chocolate agar contains both 5% Sheep blood agar only contains hemin http://gold.aecom.yu.edu/id/micro/xvfactor.htm Laboratory Diagnosis, cont. Culture • S. aureus produces NAD as a metabolic product - Haemophilus will satellite around colonies of S. aureus when growing on BAP http://www.petermp.dk/oerepodning.htm Laboratory Diagnosis, cont. Culture, cont. • Growth conditions: Haemophilus spp.: 35 – 37°C, 5-10% CO2, 3 days H. ducreyi: 33 – 35°C, 5-10% CO2, 7 days - also require supplemented media • Colony morphology: Small and translucent Exude a “mouse nest” odor http://www.uni-ulm.de/klinik/imi/mikrobio_2002/krankenversorgung/ Diagnostik/Erreger/h_keim.htm Laboratory Diagnosis, cont. Identification • Growth characterics on solid media • Gram stain morphology • X and/or V factor requirement • Satelliting • Porphyrin test Treatment Antimicrobial Susceptibility Testing and Therapy • Routine testing can be performed using disk diffusion or broth dilution • Special supplemented media required • Beta-lactamase testing routinely performed • Test panel limited because of lack of resistance to later generation cephalosporins • Cefotaxime or Ceftriaxone are drugs of choice Prevention Vaccine • Routine vaccination with protein-polysaccharide conjugated vaccine (Hib) • Significant reduction of serious, life-threatening infections in children • Recommended starting at 2 months of age CDC PHIL Bordetella sp. Organism Reservoir Transmission B. pertussis Not normal flora; only present during infection Person-to-person; airborne transmission via cough B. parapertussis Not normal flora; only present during infection Person-to-person; airborne transmission via cough B. bronchiseptica Normal flora of animal upper resp. tract (dogs, cats, rabbits) Exposure to contaminated droplets following close contact with animals Clinical characteristics B. pertussis and B. parapertussis - cause URT infections in humans with almost identical symptoms, epidemiology and therapeutic management - Pertussis (whooping cough) - optimal recovery requires special culture media B. bronchiseptica - opportunistic infection in compromised patients with history of close animal contact (pneumonia, bacteremia, UTI, meningitis, endocarditis) Clinical characteristics, cont. Epidemiology • Pertussis primarily caused by B. pertussis, rarely by B. parapertussis; former cause more severe disease - higher infection rates and increased duration of symptoms • Prior to vaccine, epidemic disease occurred in 2 – 5 cycles; still occurs in unvaccinated populations • Adults and adolescents can serve as reservoirs and transmit to unvaccinated or vaccinated with waning immunity Clinical characteristics, cont. Pathogenesis Multiple virulence factors with various functions Adhesion Fimbriae Filamentous hemagglutinin Toxicity Pertussis toxin Adenylate cyclase toxin Tracheal cytotoxin Outer membrane inhibits host lysozyme Siderophore production to circumvent host iron sequestering Clinical characteristics, cont. Spectrum of disease Catarrhal Mild cold Several weeks Paroxysmal Severe coughing “Whooping” 1 to 4 weeks Convalescent Symptoms Months Symptoms in adults tend to be milder and are misdiagnosed as bronchitis; also tend to be mixed with other pathogens Laboratory Diagnosis Specimen collection • Nasopharyngeal wash or swab (Calcium alginate or dacron on a flexible wire shaft) • Swabs should be immediately inoculated onto media and direct smears made at the bedside • Swabs not directly inoculated should be placed in transport if time to lab is extended Laboratory Diagnosis, cont. Direct detection • DFA of smear using polyclonal Abs against B. pertussis and B. parapertussis • Sensitivity is limited (50 – 70% at best), so should always be used in conjunction with culture • PCR methods (home-brew and commercial assays) are increasing in use and are replacing culture as gold standard - specificity has been an issue DFA for Bordetella ASM Color Atlas of Bacteriology Laboratory Diagnosis, cont. Culture • Historical gold standard • Selective media required Bordet-Gengou - Potato infusion agar with glycerol and sheep blood Methicillin or cephalexin Regan-Lowe - Charcoal agar with 10% horse blood Cephalexin Laboratory Diagnosis, cont. Culture, cont. • 35 – 37°C, 5 – 10% CO2, hold for 10 – 12 days - most isolates are detected in 3 – 5 days • Colonies are small, shiny; resemble mercury drops • Gram stain shows small, faintly staining gram negative coccobacilli - confirm identity with DFA reagents - can distinguish between B. pertussis and B. parapertussis B. pertussis on Regan-Lowe agar ASM Color Atlas of Bacteriology Gram stain of B. pertussis http://www2.mf.uni-lj.si/~mil/bakt2/bakt2.htm Regan-Lowe w/ antibiotics Bordet-Gengou w/o antibiotics ASM Color Atlas of Bacteriology Treatment Antimicrobial Susceptibility Testing and Therapy • Not routinely performed because Erythromycin and Azithromycin are active and remain drugs of choice Prevention Vaccine • Whole-cell vaccines have been used historically - adverse reactions and waning immunity • Acellular vaccines have been developed and include booster doses for older children and adults Neisseria and Moraxella – General characteristics • Gram-negative diplococci, oxidase-positive – Epidemiology • Table 45-1 – Pathogenesis • Table 45-2 – Other Neisseria are saprophytes Gram stain of Neisseria Gram stain of Moraxella http://www.labquality.fi/finnish/alustavat_tulokset/gramvarjays_pesake.htm – Laboratory Diagnosis • Specimen collection and transport – No special considerations for Moraxella – Pathogenic Neisseria are sensitive to drying and temp extremes – Swabs are acceptable for GC culture if plated in 6 hrs. » best method for GC culture is direct inoculation » Describe JEMBEC plates – Blood cultures as per routine, although Neisseria inhibited by high conc of SPS • Specimen processing – JEMBEC should be incubated as soon as received in lab – Body fluids should be kept at RT or 37C before culture (not cold) – Vol >1 ml should be concentrated and plate the sediment (e.g. joint fluid or CSF) – Laboratory detection • Direct detection – Gram stain » shows GN diplococci for both genera; Moraxella tend to be bigger and fatter » GNDCs in PMNs from the urethral discharge of symptomatic male is diagnostic for GC » Normal vaginal and rectal flora has GNDCs so diagnosis requires confirmation – Antigen detection » not recommended; poor sensitivity – Molecular detection » Amplified methods are more sensitive than nonamplified methods » Increased detection of GC overall » Can test for CT at the same time » Cannot be used as evidence in medico-legal cases » We use B-D Viper automated instrument – Laboratory detection • Culture – Media of choice » N. meningitidis, Moraxella and saprophytic Neisseria grow well on BAP, CAP » GC requires enriched CAP on primary culture » Selective media have been developed to inhibit normal flora and allow N. meningitidis and GC to grow » Modified Thayer-Martin » IsoVitaleX, colistin, nystatin, vanco, trimethoprim » Martin Lewis is similar – Incubation conditions and duration » 35-37C, 3 - 7% CO2, humid, 72 hrs » this CO2 conc can be achieved in incubator or candle jar – Colony appearance Culture of Neisseria http://www.bmb.leeds.ac.uk/mbiology/ug/ugteach/dental/tutorials/std/gccult.html Culture of Moraxella http://www.infek.lu.se/bakt/english/picture5.htm – Laboratory detection • Approach to identification – Biochemicals » Moraxella: glucose -, maltose -, lactose -, butyrate disk +, ox + » GC: g +, m -, l –, ox + » NM: g +, m +, l –, ox + » Saprophytes: + + + or any other combo » Culture confirm and ID must be unequivocal in abuse cases » Saprophytes are not routinely identified (i.e. from respiratory cultures – Serotyping » Mening: A, B, C, Y, W135 – Susceptibility testing and therapy • Moraxella – testing not routinely performed because many options available » beta-lactams; b-l/b-lactamase inhib; cephs; macrolides; quinolones; bactrim • GC – routinely not performed because most labs use molecular so no isolate – resistance is a Public Health issue so surveillance mechanisms exist » penicillin resistance is widespread » ceftriaxone resistance not documented » quinolone resistance is emerging problem • N. meningitidis – not routinely performed; resistance rare – pen, cephs – Prevention • Vaccine available for A, C, Y, W135 – military recruits, college students, asplenics > 2 y.o. • Chemoprophylaxis with rifampin, cipro, or ceftriaxone for close contacts of patients with meningococcal disease – no chemo prophylaxis for pneumococcal mening • Eye antibiotics for neonates to prevent gonococcal eye infections