Dabigatran
advertisement

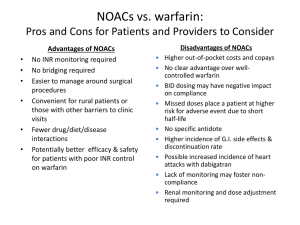
Allyson Sarigianis, Pharm.D. October 19, 2013 Review current practice recommendations for prevention of stroke/systemic embolism in atrial fibrillation Compare and contrast pharmacology between warfarin and target specific oral anticoagulants (TSOACs) Summarize the efficacy and safety of TSOACs for atrial fibrillation Examine the differences between warfarin and TSOACs for atrial fibrillation Identify future clinical implication for new era in anticoagulation management based on AT9 2012 Chest Guidelines Current FDA Approved Indications Warfarin • Prophylaxis and treatment of venous thrombosis and its extension, pulmonary embolism (PE) • Prophylaxis and treatment of thromboembolic complications associated with atrial fibrillation and/or cardiac valve replacement • Reduction in the risk of death, recurrent myocardial infarction (MI), and thromboembolic events Dabigatran • Stroke prevention in patients with non-valvular atrial fibrillation Rivaroxaban • Stroke prevention in patients with non-valvular atrial fibrillation • Prevention of VTE in patients undergoing hip or knee replacement • Acute treatment of DVT/PE • Secondary prevention of DVT/PE Apixaban • Stroke prevention in patients with non-valvular atrial fibrillation Kinetics Absorption Oral: Rapid, complete Distribution 0.14 L/kg Metabolism Hepatic, primarily via CYP2C9; minor pathways include CYP2C8, 2C18, 2C19, 1A2, and 3A4 Excretion Urine (92%, primarily as metabolites) Half-life 20-60 hours Onset of action: 5-7 days May requiring bridging Antidote: Vitamin K, FFP, PRBC Interactions: Foods with high vitamin K content Prednisone Medications Rifampin Amiodarone SSRIs Antiplatelets Sulfonamides (Bactrim) Azole antifungals (fluconazole) 2nd/3rd-gen Cephalosporins Fluoroquinolones (ciprofloxacin) Tetracyclines (Doxycycline ) Herbals Griseofulvin Ginger Isoniazid Gingko Macrolides (clarithromycin) Fenugreek Metronidazole Chamomile NSAIDs St. John’s Wort Penicillins (nafcillin) ADRs Bleeding/Hemorrhage/Hematuria Vasculitis Dermatitis, pruritus, urticaria Abdominal pain, N/V/D Anemia Skin necrosis, gangrene, “purple toes” syndrome MOA: direct thrombin inhibitor which inhibits: Both free and fibrin-bound thrombin Cleavage of fibrinogen to fibrin Activation of factors V, VIII, XI, and XIII Thrombin-induced platelet aggregation Kinetics Absorption Rapid; initially slow postoperatively Distribution Vd: 50-70 L Metabolism Hepatic; rapidly and completely hydrolyzed to active form by plasma and hepatic esterases Excretion Renal (80%) Half-life 12-17 hours Monitoring PPT Onset: 1 hour, delayed by food Antidote: None ADRs Bleeding (8% to 33%; major ≤ 6%) Dyspepsia (11%) Contraindications Hypersensitivity to dabigatran or any component Active bleeding Warnings/Precautions Bleeding Renal impairment Anticoagulants Invasive/surgical invasions P-gp inducers/inhibitors Drug interactions Category X: P-Gp inducers Category D: Amiodarone, P-Gp inhibitors, quinidine, St. john’s Wort, verapamil Category C: antacids, anticoagulants, antiplatelet agents, atorvastatin, dasantinib, ibritumonmab, NSAIDs, prostacyclin analogs, PPIs, salicylates, thrombolytic agents FDA Bleeding Risk: [12-7-2011] Evaluating post-marketing reports of serious bleeding “Bleeding that may lead to serious or even fatal outcomes is a well-recognized complication of all anticoagulant therapies.” ISMP Medication Safety Alert: Quarter Watch [01-12-12] 932 serious adverse events for 1st quarter of 2011 120 deaths 25 cases of permanent disability 543 cases requiring hospitalization 505 cases involved hemorrhage: elderly patients (Median age of 80) ▪ 120 cases of hemorrhagic stroke FDA Drug Safety Communication: [11‐02‐2012] “… FDA investigated the actual rates of gastrointestinal bleeding and intracranial hemorrhage for new users of [dabigatran] compared to new users of warfarin. The results of this Mini‐Sentinel assessment indicate that bleeding rates associated with new use of [dabigatran] do not appear to be higher than bleeding rates associated with new use of warfarin ….” 18 FDA Drug Safety Communication: [12-19-2012] “A clinical trial in Europe (the RE-ALIGN trial) was recently stopped because [dabigatran] users were more likely to experience strokes, heart attacks, and blood clots forming on the mechanical heart valves than were users of the anticoagulant warfarin. There was also more bleeding after valve surgery in the [dabigatran] users than in the warfarin users [dabigatran] is not approved for patients with AF caused by heart valve problems. “ 19 May be appropriate Ability to comply with twice daily drug regimen Unstable INRs on warfarin (unrelated to adherence) Difficulty obtaining regular INRs on warfarin Complicated interacting drug regimens on warfarin MAY NOT be appropriate NOT appropriate History of non-adherence Severe renal impairment (CrCl <30 ml/min) Stable INRs on warfarin History of GI bleeding or recent ulcers Advanced age (75-80 yrs and older; consider benefits and risks) Active liver disease Pregnancy, at risk of pregnancy, or lactating High risk of intracranial bleed Need for concomitant treatment with P-gp inducer (e.g,. rifampin, St. John’s Wort) Medication regimen does not include drugs that interact with dabigatran Moderate renal impairment (CrCl 3050 ml/min) and the need for concomitant treatment with the P-gp inhibitors dronedarone or systemic ketoconazole Good renal function No history of GI bleeding or recent ulcers MOA: selective/reversible direct inhibitor of factor Xa Prevents the conversion of prothrombin to thrombin Thrombin both activates platelets and catalyzes the conversion of fibrinogen to fibrin Creatine Clearance (mL/min) Atrial fibrillation >50 20 mg po daily 50-30 29-15 15 mg po daily <15 or HD Avoid use Postoperative thromboprophylaxis 10 mg po daily Knee: 12-14 days Hip: 35 days 10 mg po daily, use with caution Avoid use Avoid use Treatment of PE/VTE 15 mg twice daily x21 days, then 20 mg po daily Use with caution Avoid use Avoid use Secondary Prophaxis for PE/VTE 20 mg po daily Use with caution Avoid use Avoid use Kinetics Absorption Rapid Distribution Vdss: ~50 L Metabolism Hepatic (33%) via CYP3A4/5 and CYP2J2 Excretion Renal (66% primarily via active tubular secretion); feces (28%) Half-life 5-9 hours Monitoring Prothrombin time (PT) CBC with differential Renal/hepatic function Onset: 2-4 hours Antidote: None ADRs Pruritus (2%) Bleeding ▪ DVT prophylaxis: 6% [major: <1%] ▪ Atrial fibrillation: 21% [major: 6%] Thrombocytopenia (3%) Increase in liver enzymes (7%-3%) Contraindications Hypersensitivity to rivaroxaban or any component Active bleeding Drug Interactions Category X: P-Gp or 3A4 inhibitors/inducers Category C: anticoagulants, antiplatelet agents, NSAIDs, salicylates ISMP Medication Safety Alert: 10/4/2012 Primary event: Thrombus 158 cases, 44.4% of the total Hemorrhage 121 cases, 34% of the total MOA: oral direct Xa inhibitor Dose: 5mg twice daily Dose reduction to 2.5mg twice daily if 2+ of the following: ▪ Age ≥80 years ▪ Body weight ≤60kg ▪ Scr ≥1.5mg/dl AVOID in CrCl <15 ml/min Kinetics Absorption Rapid; Intestines Distribution Vd: 21 L Metabolism 15% liver metabolism CYP3A4/5 P-gp Excretion Primarily Biliary/Fecal (46-56%) Renal (27%) unchanged 8 to 15 hours Half-life Monitoring Minimal impact on the PT, INR, or aPTT Factor Xa inhibition Onset: 3-4 hours Antidote: None RE-LY ROCKET-AF ARISTOTLE Dabigatran 150mg BID vs. warfarin Rivaroxaban 20mg daily vs. warfarin Apixaban5mg BID vs. warfarin Study Design Trial design Sample size (n) RCT Open blinded assessment RCT DB DD RCT DB DD 18,000+ 14,000+ 18,000+ Inclusion criteria AF and selected risk factor(s) for embolization AF and CHADS2 ≥2 AF or flutter and CHADS2 ≥1 Key exclusion criteria Valvular AF Use of ASA ≥100 mg/day CrCl <30 ml/min Valvular AF; Use of ASA >100 mg/day CrCl <30 ml/min Valvular AF Need for ASA >165 mg/day SCr >2.5mg/dL or CrCl <25ml/min Follow-up (mean) Primary Efficacy Major Bleeding Mortality Age (years) Female (%) CHADS2 (mean) Previous embolic episode (%) TTR (%) (Standard 60-65%) 2 yr 1.9 yr 1.8 yr Outcome Definitions Composite of systemic embolism and stroke (ischemic or hemorrhagic) ISTH: fatal/critical organ bleed; decrease ≥2g/dL Hbg or transfusion of ≥2U blood All causes Baseline Characteristics 71 (mean) 73 (median) 70 (median) 36.4% 39.7% 35.2 % 2.1 3.5 2.1 20% 55% 19% (stroke or TIA only) (stroke,TIA, systemic embolism) (stroke, TIA, systemic embolism) 64% 55% 62% Comparison of Efficacy Results RE-LY Outcome (%/year) Dabigatran 150mg BID vs. warfarin Primary Outcome Stroke or systemic embolism Stroke ROCKETAF p Value Apixaban 5mg BID vs. warfarin p Value 2.1 vs. 2.4% p=0.12 1.3 vs. 1.6% p=0.01 NNT 167 p<0.001 NNT 88 1.65 vs. 1.96% p=0.09 1.2 vs. 1.5% p=0.01 NNT 175 0.9 vs. 1.3% p=0.03 NNT 132 1.3 vs. 1.4 p=0.58 0.97 vs. 1.05% p=0.42 0.1vs0.4% p<0.001 NNT 182 0.26 vs. 0.44% p=0.02 NNT 333 0.24 vs. 0.47% p<0.001 NNT 238 All cause death 3.6 vs. 4.1% p=0.051 4.5 vs. 4.9% p=0.15 3.5 vs. 3.9 p=0.047 NNT 132 MI/ACS 0.7 vs. 0.5% p=0.048 NNH 239 0.9 vs. 1.1% p=0.12 0.5 vs. 0.6% p=0.37 Ischemic stroke Hemorrhagic stroke p Value Rivaroxaban 20mg daily vs. warfarin 1.1 vs. 1.7% p<0.001 NNT 88 1.0 vs. 1.6% ARISTOTLE Comparison of Safety Results RE-LY Major bleed 3.1 vs. 3.36% Intracranial bleed GI bleed ROCKETAF p=0.31 ARISTOTLE 2.1 vs. 3.1% p<0.001 NNT 67 0.5 vs. 0.7% p=0.02 NNT 250 0.3 vs. 0.8% p<0.001 NNT 128 3.2 vs. 2.2%** p=0.001 NNH 100 0.76 vs. 0.86% 0.37 3.6 vs. 3.4% p=0.58 p<0.001 0.3 vs. 0.74% NNT 116 p<0.001 1.5 vs. 1.0% NNH 100 Focused update recommendation: Dabigatran is a useful alternative to warfarin for the prevention of stroke and systemic embolism in patients with paroxysmal to permanent AF and risk factors for stroke and systemic embolism Do not have a prosthetic heart valve, hemodynamically significant valve disease, severe renal failure (CrCl < 15mL/min), or impaired liver disease Alternative to warfarin for moderate-high risk patients: ▪ ▪ ▪ ▪ Difficulty achieving therapeutic INRs Inability obtaining regular bloodwork monitoring Low risk for GI bleeding Low risk for cardiovascular events 2.1.8 CHADS = 0 No therapy rather than antithrombotic therapy (Grade 2B) 2.1.9 CHADS = 1 Oral anticoagulation rather than no therapy (Grade 1B) 2.1.10 CHADS =2+ Oral anticoagulation rather than no therapy (Grade 1A) 2.1.11 Dabigatran 150 mg twice daily rather than adjusted-dose VKA therapy (target INR range, 2.0-3.0) (Grade 2B) Dabigatran Rivaroxaban Apixaban Factor IIa Factor Xa Factor Xa Nonvalvular AF Nonvalvular AF Ortho VTE Proph Acute Treatment VTE Nonvalvular AF Prodrug Yes No No Dosing Twice daily Daily, with food Twice daily Onset 1-2 hrs 2-4 hrs 3-4 hrs Half-life (h) 14–17 7–11 8–14 Renal Adjustment ↓ 15-29ml/min Avoid < 15 ml/min Avoid < 30 ml/min Avoid < 15 ml/min Drug Interactions P-gp CYP3A4/P-gp CYP3A4/P-gp Target FDA Indications Efficacy superiority vs non- inferiority ADRs ACS / MI risk Cost Clinical guidelines Antibiotic/Antifungal Drug Interactions and Warfarin. Pharmacist's Letter 2012; 28(1):280102. Connolly SJ, Ezekowitz MD, Yusuf S, et al. RELY Trial: Dabigatran versus warfarin in patients with atrial fibrillation. NEJM.2009; 361(12): 1139-51. Wallentin L, Yusuf S, Ezekowitz MD, et al. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalized ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010; 376: 975-83. Reversing Dabigatran and Rivaroxaban. Pharmacist's Letter 2011; 27(9):270912. Weitz JI. New oral anticoagulants in development. Thromb Haemost 2010; 103: 62–70. Institute of Safe Medication Practices. ISMP Medication Safety Alert, January 12, 2012. “Outpatient Anticoagulation Management” VA Medical Center: MCM 11-20. Updated August 2011. Dabigatran (Pradaxa®) National Drug Monograph, VA Pharmacy Benefits Management Services, Medical Advisory Panel, and VISN Pharmacist Executives, April 2011. “Dabigatran etexilate: drug information.” www.uptodate.com. Accessed 20 January , 2012. Rivaroxaban. Monograph. Medscape. Accessed 24 January, 2012. http://reference.medscape.com/drug/xarelto-rivaroxaban999670#10 Patel MA, Mahaffey KE, Garg JY, et al. Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation. N Eng J Med. 2011. Lassen MR, Raskob GE, Gallus A, et al. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594-604. Lopes RD, Alexander JH, Al-Khatib SM, et al; ARISTOTLE Investigators. Apixaban for reduction in stroke and other thromboembolic events in atrial fibrillation (ARISTOTLE) trial: design and rationale. Am Heart J. 2010;159:331-9. Eikelboom JW, O’Donnell M, Yusuf S, et al. Rationale and design of AVERROES: apixaban versus acetylsalicylic acid to prevent stroke in atrial fibrillation patients who have failed or are unsuitable for vitamin K antagonist treatment. Am Heart J. 2010;159:348-53. Comparison of Oral Antithrombotics. Pharmacist's Letter. 2012; 28(1):280102. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation 2012; 126:2381. Guyatt GH , Akl EA, Crowther M, et al. Antithrombotic Therapy and Prevention of Thrombosis.. American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (9th Edition). Chest 2012; 141:7S-47S