N Engl J Med 2011
advertisement

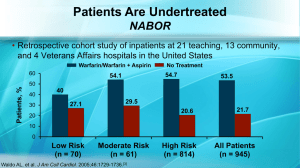
Stroke Prevention in Atrial Fibrillation An Expert Commentary With Michael D. Ezekowitz, MD, PhD A Clinical Context Report Stroke Prevention in Atrial Fibrillation Expert Commentary Jointly Sponsored by: and Stroke Prevention in Atrial Fibrillation Expert Commentary Supported in part by an educational grant from Ortho-McNeil, Division of Ortho-McNeilJanssen Pharmaceuticals, Inc., administered by Ortho-McNeil Janssen Scientific Affairs, LLC. Stroke Prevention in Atrial Fibrillation Clinical Context Series The goal of this series is to provide up-todate information and multiple perspectives on the pathogenesis, symptoms, risk factors, and complications of stroke prevention in atrial fibrillation as well as current and emerging treatments and best practices in the management of stroke prevention in atrial fibrillation. Stroke Prevention in Atrial Fibrillation Clinical Context Series Target Audience Electrophysiologists, cardiologists, primary care physicians, nurses, nurse practitioners, physician assistants, pharmacists, and other healthcare professionals involved in the management of stroke prevention in atrial fibrillation. Activity Learning Objective CME Information: Physicians Statement of Accreditation This activity has been planned and implemented in accordance with the Essential Areas and Policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of the University of Pennsylvania School of Medicine and MedPage Today. The University of Pennsylvania School of Medicine is accredited by the ACCME to provide continuing medical education for physicians. CME Information Credit Designation The University of Pennsylvania School of Medicine Office of CME designates this enduring material for a maximum of 0.5 AMA PRA Category 1 Credits.™ Physicians should claim only the credit commensurate with the extent of their participation in the activity. CME Information: Physicians Credit for Family Physicians MedPage Today "News-Based CME" has been reviewed and is acceptable for up to 2098 Elective credits by the American Academy of Family Physicians. AAFP accreditation begins January 1, 2011. Term of approval is for one year from this date. Each article is approved for 0.5 Elective credit. Credit may be claimed for one year from the date of each article. CE Information: Nurses Statement of Accreditation – Projects In Knowledge, Inc. (PIK) is accredited as a provider of continuing nursing education by the American Nurses Credentialing Center’s Commission on Accreditation. – Projects In Knowledge is also an approved provider by the California Board of Registered Nursing, Provider Number CEP-15227. – This activity is approved for 0.5 nursing contact hours. DISCLAIMER: Accreditation refers to educational content only and does not imply ANCC, CBRN, or PIK endorsement of any commercial product or service. CE Information: Pharmacists Projects In Knowledge® is accredited by the Accreditation Council for Pharmacy Education (ACPE) as a provider of continuing pharmacy education. This program has been planned and implemented in accordance with the ACPE Criteria for Quality and Interpretive Guidelines. This activity is worth up to 0.5 contact hours (0.05 CEUs). The ACPE Universal Activity Number assigned to this knowledge-type activity is 0052-9999-11-2353-H01-P. Discussant Michael D. Ezekowitz, MD, PhD Professor of Medicine Cardiovascular Medicine Mainline Healthcare Interventional Cardiology Wynnewood, Pennsylvania Disclosure Information Michael D. Ezekowitz, MD, PhD, has disclosed the following relevant financial relationships: Served as a consultant for: – – – – – – – – – – – – – ARYx Therapeutics AstraZeneca Pharmaceuticals Boehringer Ingelheim Pharmaceuticals, Inc. Bristol-Myers Squibb Daiichi-Sankyo Eisai Inc. Gilead Science, Inc. Johnson & Johnson Medtronic, Inc. Merck & Co., Inc. Pfizer Inc. Portola Pharmaceuticals Sanofi Disclosure Information Michael Mullen, MD, Clinical Instructor of Vascular Neurology, University of Pennsylvania; Todd Neale; and Dorothy Caputo, MA, RN, BC-ADM, CDE, Nurse Planner, have disclosed that they have no relevant financial relationships or conflicts of interest with commercial interests related directly or indirectly to this educational activity. The staff of The University of Pennsylvania School of Medicine Office of CME, MedPage Today, and Projects In Knowledge have no relevant financial relationships or conflicts of interest with commercial interests related directly or indirectly to this educational activity. Risk Factors for Stroke in Atrial Fibrillation • Previous stroke or TIA • Older age • Hypertension • Diabetes • Heart failure • Female gender • Vascular disease Warfarin Era Completed Studies: Warfarin vs. Placebo OPEN LABEL # Events AFASAK: Peterson, et al Pt-yrs Lancet 1989; 1: 175 27 811 BAATAF: Investigators 15 922 23 508 29 972 NEJM 1990; 323: 1505 SPAF: Investigators Stroke 1990; 21: 538 DOUBLE BLIND SPINAF: Ezekowitz, et al NEJM 1992; 327: 1406 100% 50% 0% Warfarin better -50% -100% Warfarin worse Risk Reduction Modern Era: RE-LY 150 mg BID Source: Connolly S, et al ”Dabigatran versus wafarin in patients with atrial fibrillation” N Engl J Med 2009: 361.c & N Engl J Med 2010: 363. Modern Era: ARISTOTLE Source: Connolly S, et al ”Dabigatran versus wafarin in patients with atrial fibrillation” N Engl J Med 2009: 361.c & N Engl J Med 2010: 363. Granger, et al N Engl J Med 2011 Rivaroxaban versus WARFARIN (ROCKET-AF) Efficacy Outcomes Stroke/Systemic Embolism Hemorrhagic Stroke Myocardial Infarction Safety Outcomes ICH Major Bleeding 0 0.50 1.00 Rivaroxiban better 1.50 Warfarin better 2.00 Patient Populations Lacking Data With New Anticoagulants • Patients with mechanical heart valves • Patients with poor renal function • Children Reduction in Intracranial Hemorrhage Versus Placebo • Dabigatran 150 mg BID – 0.30% versus 0.74% (RR 0.40, P<0.001) • Apixaban 5 mg BID – 0.33% versus 0.80% (HR 0.42, P<0.001) • Rivaroxaban 20 mg – 0.5% versus 0.7% (HR 0.67, P=0.02) Sources: N Engl J Med 2009; 361: 1139-1151; N Engl J Med 2011; 365: 883-891; N Engl J Med 2011; 365: 981-992. Mortality Reductions Versus Placebo • Dabigatran 150 mg BID – 3.64% versus 4.13% (RR 0.88, P=0.051) • Apixaban 5 mg BID – 3.52% versus 3.94% (HR 0.89, P=0.047) • Rivaroxaban 20 mg – 4.5% versus 4.9% (HR 0.92, P=0.15) Sources: N Engl J Med 2009; 361: 1139-1151; N Engl J Med 2011; 365: 883-891; N Engl J Med 2011; 365: 981-992. Summary At the end of this activity, participants should understand: Strokes associated with afib tend to be severe, killing about 20% of patients in a month 60% of survivors are severely disabled Afib-related strokes tend to become more common as the population ages Summary Dabigatran (Pradaxa) is a direct thrombin inhibitor, and apixaban and rivaroxaban (both not yet approved) are direct factor Xa inhibitors All have been shown to as effective (rivaroxaban) or better (dabigatran and apixaban) than warfarin at preventing strokes It is unclear whether the different mechanisms of action will be important in differentiating between the new anticoagulants Summary Warfarin will remain relevant, as some patient populations – including those with mechanical heart valves – have not been included in the trials of new anticoagulants Patients who are well controlled on warfarin might want to keep taking it because it is inexpensive Conversely, the reduction in intracranial bleeding with the newer anticoagulants might argue for switching patients who are well controlled on warfarin Summary Patients must be committed to taking the new anticoagulants and to the twice-daily regimen Emphasis must be placed on minimizing temporary and permanent discontinuation of the novel anticoagulants Much of the bleeding risk with the new anticoagulants comes from extracranial bleeds, which are more tolerable than intracranial hemorrhages