UMSSci823to312012 - Randolph County Schools

1)Objective: I will learn about the procedures that will be set to ensure a successful semester of learning 8 th
Grade Science.
2) Essential Question: What procedures do I need to remember in order to be successful in science.
3) Warm-up: Two paragraphs. What do you remember learning in 6 th and 7 th
Grade Science. What helped you to remember it?
4) Vocabulary: order, structure
Student Information Sheet
8 th Grade Science Introduction
Tell Mr. Harvey about yourself
Engrade information and login
1)Objective: I will learn about the classification of matter.
2) Essential Question: How can I explain the different classifications of matter?
3) Warm-up: 2 paragraphs- Explain in detail each of the three states of matter.
4) Vocabulary: Matter, atom, element, compound
1) Matter= Anything that has mass and occupies space
2) Element- A pure substance that can not be broken down into another substance by chemical or physical means
3) Atom= the basic particle from which all elements are made
4) compound= A pure substance made of two or more chemically combined elements.
Lab Zone Discover Activity p. 604
Read p. 604-609
Answer the Section Assessment questions on p. 609
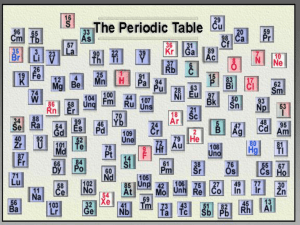
1)Objective: I will learn how the elements on the periodic table are arranged.
2) Essential Question: Can I identify how the elements on the
Periodic Table are arranged?
3) Warm-up: Identify the mass of protons, electrons, and neutrons.(p.
607.
4) Vocabulary: nucleus, protons, electrons, neutrons
1)
2)
Nucleus= the very small center core of an atom.
Proton- a positively charged atomic particle, found in the atom’s nucleus
3) electron- a negatively charged atomic particle, found outside the atom’s nucleus.
4) neutron- an atomic particle with no charge, found inside the atom’s nucleus
Lab Zone Discover Activity p. 610
Read p. 609-617
1)Objective: I will learn about the different properties of elements on the periodic table.
2) Essential Question: How would
I explain the properties of different elements on the periodic table
3) Warm-up: Create a circle web of the different models of atoms from
1808 to the present. P. 606-607
4) Vocabulary: atomic mass, atomic number, periodic table, isotope
1 )
Atomic mass number= appears below the chemical symbol of an element. Is always equal to the sum of protons and neutrons in an atom’s nucleus
2)Atomic number= appears above the chemical symbol of an element. Is equal to the number of protons in an atom’s nucleus
3) Periodic table= A chart of the elements showing the repeating pattern of their properties.
4) isotope= An atom with the same number of protons but a different number of neutrons from the most stable form of an element.
Vocabulary quiz Friday on the following 20 words:
Matter, element, atom, nucleus, neutrons, protons, electrons, atomic number, atomic mass number, isotope, periodic table, periodic group, ductility, reactivity, corrosion, malleability, alloys, conductivity, magnetism, semiconductor
1)
2)
3)
4)
What information did Mendeleev use to find a pattern in the elements?
When did Mendeleev notice a pattern?
Why was Mendeleev able to predict the properties of elements that had not yet been discovered?
What information about an element is given in each square of the periodic table?
1) Melting point, .density, color, atomic mass, and the number of chemical bonds it could form.
2) When he arranged the elements in order of increasing atomic mass
3) He noticed a repeating pattern of the elements
4) Atomic number, chemical symbol, name, atomic mass
5) From the information in each square, what do you know about the structure of the element?
6) What are the rows of the periodic table called?
7) How do the properties of the metals in a period change from left to right?
8) What are the columns in the periodic table called?
5) The number of protons and electrons and the average number of neutrons
6) periods
7) From highly reactive to very unreactive
8) groups. By looking at an element’s periodic group, you can find other elements with similar properties
Read p. 609-617
1)Objective: I will learn about the different properties elements may have.
2) Essential Question: Can I evaluate the significance of the different properties of elements
3) Warm-up: How is the modern day periodic table different than the one created by Dmitri Mendeleev?
4) Vocabulary: ductility, malleability, conductivity, reactivity, magnetism
1) Malleability= the ability of an element to be rolled into thin sheets.
2) ductility= the ability of an element to be stretched into wire
3) conductivity= the ability of an element to transfer heat or electricity to another object.
4) reactivity= the ease and speed with which an element combines with other substances
5) magnetism= the ability of an element to attract other materials.
1)
Questions on Notes
Where would you look on the periodic table to find an element with properties similar to that of another element?
2) What four things are found in each square of the periodic table?
3) What five things did Dmitri Mendeleev use to find a pattern in the elements?
4) What are the horizontal rows of the periodic table called?
5) How do the properties of metals in a period change from left to right?
Lab Zone Discover Activity p. 618
Group Reading p. 618-625
Review and Reinforce Worksheet packet
Write one paragraph evaluating the importance of the different properties elements have. How do we depends on these properties in our everyday life?
www.reviewgamezone.com
Codes:10229, 10230
1)Objective: I will learn about the use of metals in everyday life.
2) Essential Question: How well can I identify the use of metals in everyday life?
3) Warm-up: Answer the Math
Analyzing Data questions on page 621.
4) Vocabulary: corrosion, alloy, semiconductor, periodic group
1)
2)
3)
4) corrosion= The gradual wearing away of a metal due to a chemical reaction. (example: rust)
Alloy= the mixing of two elements one of which is a metal. (Example: alloy rims on cars)
Semiconductor= An element that conducts current under certain conditions. Example: the use of silicon in cellphones and other electronics.
Periodic group= the horizontal rows of the periodic table, arranged based on their similar properties. (example: by looking at an element’s periodic group, you can find other elements with similar properties)
Scavenger Hunt for Metals-
Write down a list of items throughout the school that have metals. Can you identify the metals that are being used?
Labzone Discover Activity p. 628
Review and Reinforce Worksheet packet
www.reviewgamezone.com
Codes:10229, 10230
Write one paragraph evaluating the importance of the metals. How do we depend on these metals in our everyday life?
1)Objective: I will apply the concepts I have learned this week through by using my inferencing skills to decipher context clues.
2) Essential Question: How well do
I understand the vocabulary words
I learned in class this week?
3) Warm-up: 5 minutes of study time for your vocabulary quiz
4) Vocabulary review: Atoms, elements, periodic table
Tips for Success on Today’s Vocabulary Quiz
1)
2)
Think of each statement in the way it would be said by someone.
Think of the hints (underlined words) in the statement that give away the vocabulary word.
3)
4)
Read all of each statement first and match the statement with the word that best fits.
I believe in you. I know you will do well.
Believe in yourself also and you will be amazed.
Co ngratulations! You are about to have the grand opening for your first business. You have decided to open up a store of metals. It’s time to get to work so you can load the store full of customers for the grand opening. Think of the types of metals you will sell and what they might be useful for. You may refer to the list of metal objects you found in the scavenger hunt yesterday.
Steps for creating the store of metals
1) Identify 5 metals your store will sell.
2) Draw illustrations depicting what the metals you sell may be used for.
3) Come up with a name for your store of metals. Create a propaganda slogan to market your store to the public
4) Prepare a commercial to present to the class advertising your store.