Elements and the Periodic Table
advertisement

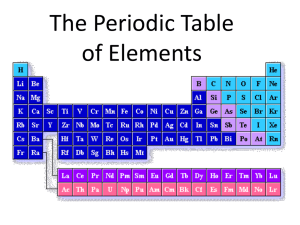
Elements and the Periodic Table Chapter 5 5.1 - Journal 1. Take out your homework 2. Color in the Periodic Table given to you and color it in according to the following LIST. Use your book, too! Use a different color for each of the items below. Alkali Metals Alkaline Earth Metals Transition Metals Metalloids Nonmetals Halogens Noble Gases Other metals 5.1 – Journal 1. Check your Periodic Table 5.1 - Organizing the Elements MENDELEEV’S ORDER Dmitri Mendeleev Trying to organize the 63 known elements so they were easier to understand 5.1 - Organizing the Elements MENDELEEV’S ORDER: Didn’t yet know about protons, neutrons and electrons, ONLY MASS.. What could he do? 5.1 - Organizing the Elements MENDELEEV’S ORDER: Increasing Atomic Mass Elements with similar properties were put in the same column 5.1 - Organizing the Elements Named “Periodic Table” because properties repeated ‘periodically’ from row to row. Like keys on a keyboard (octaves) 5.2 – The Modern Periodic Table The Periodic Law Once protons were discovered, what were elements organized by? Increasing Atomic Number. Properties ARE STILL grouped by column. So, Properties STILL repeat periodically (within a row). 5.2 – The Modern Periodic Table Like Cards in a deck: In the Modern table a PERIOD = a ROW. How many PERIODS are there? 7 PERIODS = ROWS How many ELEMENTS are in PERIODS 1, 2, and 3? What does this remind you of? Numbers of electrons in each ENERGY LEVEL a COLUMN = a GROUP ELEMENTS within a GROUP have similar PROPERTIES. COLUMNS = GROUPS Therefore, properties repeat PREDICTABLY and PERIODICALLY. THIS IS CALLED PERIODIC LAW. 5.2 – The Modern Periodic Table Example Group (Family 1) – Alkali Metals What is similar? 5.2 – The Modern Periodic Table There are 4 important pieces of information for each element Atomic number Element symbol Element name Atomic mass 5.2 – The Modern Periodic Table - Journal 1. What happens to properties when you move from left to right across the table? 2. What type of elements are in yellow? In green? In blue? 3. What is the definition of a “metalloid”? 4. What type of elements are in the “B” columns? 5. List the names of all of the “A” groups (families). 6. What is an important fact about the Actinide series? 5.2 – The Modern Periodic Table HOW CAN WE CLASSIFY ELEMENTS? 2. State: solid, liquid, gas Natural & manmade: (91 of the first 92 are natural) 3. METALS, NONMETALS & METALLOIDS 1. 5.2 – The Modern Periodic Table Classified by Properties What happens to properties when you move from left to right across the table? 5.3 – Representative Groups Elements in a group have SIMILAR (not identical) PROPERTIES because they have the same number of valence electrons 5.3 – Representative Groups ALKALI METALS (group 1): Single valence electron EXTREMELY REACTIVE Reactivity increases from top to bottom It is not a metal, but Hydrogen is grouped here. Why? 5.3 – Representative Groups ALKALINE EARTH METALS (group 2): TWO valence electrons Less reactive than group 1 5.3 – Representative Groups BORON FAMILY (group 3A): THREE valence electrons FYI- Aluminum is the most abundant metal in Earth’s crust 5.3 – Representative Groups CARBON FAMILY (group 4A): FOUR valence electrons FYI- Except for water, most compounds in your body contain Carbon. 5.3 – Representative Groups NITROGEN FAMILY (group 5A): FIVE valence electrons FYI- Nitrogen and Phosphorous are used to make fertilizers. 5.3 – Representative Groups OXYGEN FAMILY (group 6A): SIX valence electrons FYI- Oxygen is the most abundant nonmetal in Earth’s crust, and Ozone is another form of oxygen. 5.3 – Representative Groups THE HALOGENS (group 7A): SEVEN valence electrons EXTREMELY REACTIVE Reactivity increases from bottom to top FYI- Halogen means “salt former”. 5.3 – Representative Groups THE NOBLE GASES (group 8A): EIGHT valence electrons INERT (don’t react easily) FYI- make “neon” lights when a current is passed thru them.