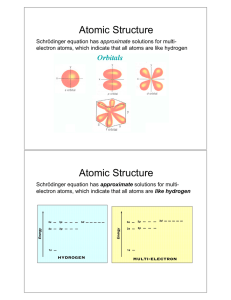
WAVE MECHANICS AND ELECTRON DISTRIBUTION THREE LAWS:
advertisement

SCH 4U1 WAVE MECHANICS AND ELECTRON DISTRIBUTION ELECTRON DISTRIBUTION IN SHELLS ARE GOVERNED BY THREE LAWS: AUFBAU PRINCIPLE AS ELECTRONS FILL THE ORBITALS, THEY START WITH THE ORBITALS OF LOWEST AVAILABLE ENERGY. PAULI EXCLUSION PRINCIPLE AN ORBITAL CAN BE EMPTY, HAVE ONE ELECTRON OR AT THE VERY MOST HAVE TWO ELECTRONS. IF AN ORBITAL HAS TWO ELECTRONS, THEY MUST HAVE OPPOSITE SPIN. HUND'S RULE ELECTRONS IN THE SAME SUB-LEVEL WILL HAVE THE SAME SPIN AND WILL NOT PAIR UP (OCCUPY THE SAME ORBITAL) UNLESS THEY MUST. * COMPLETELY FILLED SUBLEVELS HAVE GOOD STABILITY ** HALF-FILLED SUBLEVELS ALSO HAVE GOOD STABILITY *** IT IS EASIER TO REMOVE ELECTRONS FROM SMALL FILLED SUBLEVELS THAN FROM LARGE FILLED SUBLEVELS **** WHEN ELECTRONS ARE REMOVED (DURING IONIZATION) THEY ARE USUALLY REMOVED FROM THE ORBITALS OF HIGHEST ENERGY LEVEL.