Ionic Compounds Intro
advertisement

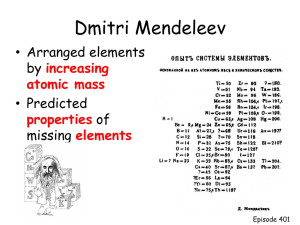
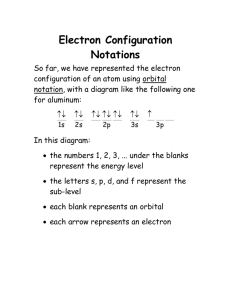
Ionic Compounds and their Properties What are Ionic Compounds Ionic compounds are compounds that are composed of cations (positively charged ions) and anions (negatively charged ions). They usually involve a metal (forms the cation) and a nonmetal (anion) What causes atoms to form ions? Every element wants to be like a noble gas and have 8 valance electrons (Rule of octet). Elements will form chemical bonds to have 8 by either sharing or transferring electrons. Ionic bonds deal with the transfer of electrons and the attractive forces between the opposite charged ions that form. Every element that is close to having 8 valance electrons will steal or give away electrons to get to 8. So, it boils down to stability Atoms can form multiple ions based on the stability of their electron configurations. The charges are known as the ion’s oxidation numbers. Remember, only ions have oxidation numbers. All atoms, elements, molecules, and compounds have an oxidation number of zero. (They are overall neutral) Ions can have oxidation numbers ranging from -4 to +7. Let’s look at Na and Cl What is the electron configuration of Na? 1s2 2s2 2p6 3s1 or [Ne] 3s1 How can Na become like a noble gas? It can lose an electron. What is the electron configuration of Cl? 1s2 2s2 2p6 3s2 3p5 or [Ne] 3s2 3p5 How can Cl become like a noble gas? It can gain an electron Let’s look at Na and Cl Na becomes What is the electron configuration of Na? Na+ and more stable 1s2 2s2 2p6 3s1 or [Ne] How can Na become like a noble gas? It can lose an electron. What is the electron configuration of Cl? 1s2 2s2 2p6 3s2 3p5 or [Ne] 3s2 3p6 How can Cl become like a noble gas? It can gain an electron [Ar] Cl becomes Cl- and more stable This can also be done with Lewis Dot Formulas So, let’s practice that… (back of homework from last class) To find the oxidation number of the ions that compose an ionic compound, remember… The elements from group 1 will be +1, group 2 will be +2, group 13 will be +3, group 15 will be -3, group 16 will be -2, and group 17 will be -1. The transitional elements vary. When dealing with two atoms, the first atom listed will have a positive number, the second will have a negative number. The sum of the charges times their subscripts must equal zero. What column does every element want to be like? Most Common Predicted Oxidation Numbers 0 +1 +2 +3 vary -3 -2 -1 Find the oxidation number for each atom in the following molecules NaCl MgO WS3 AlN ZnBr2 PCl5 HgS +1 +2 +6 +3 +2 +5 +2 -1 -2 -2 -3 -1 -1 -2 Homework Page 165: 6,7,10,11,13 and Page 183: 7,9,12,13,16