What do the quantum numbers l and m determine
advertisement

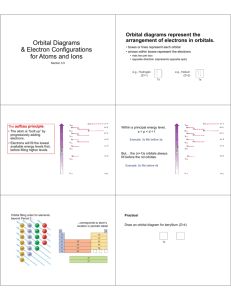
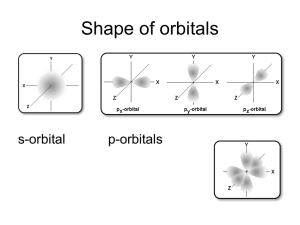
What do the quantum numbers l and m determine? l determines the shape of the orbitals and the magnitude of the angular momentum of the electron. m determines the orientation of the orbital and the direction of the angular momentum. l = 0, the distribution of the electron is spherical about the nucleus. Called an ‘s’ orbital (only one of them as m = 0). n = 1, l = 0: 1s orbital; z n = 2, l = 0, 2s; etc. (Zero angular momentum, no ‘choice’ of orientation.) l = 1 , the distribution of the electron is a ‘dumbbell’ shape. Called y ‘p’ orbitals (there are 3 of them as m = -1,0,1 or x, y, z). n = 2, l = 1, 2p x 1s z z x 2px x x y y z y 2py 2pz (Electron has angular momentum that can be directed in one of three direction.) l = 2, double dumbbell, ‘d’ orbitals. n = 3, l = 2, 3d orbitals 5 of them as m = -2, -1, 0, 1, 2 or z2, x2-y2, xz, yz, xy Note: l = 1, m = -1, 0, 1 or m = x, y, z these are equivalent but not identical. Similar l = 2, m = -2, -1, 0, 1, 2 or z2, x2-y2, xz, yz, xy are related but not identical sets of 5 functions. Hydrogen atom is a very simple system which is why it has so many degenerate orbitals. Quantum mechanics of other atoms shows one additional feature. The energy now depends on n and l. For a given n the energy increases with increasing l. 2s < 2p 3s < 3p <3d 4s < 4p < 4d < 4f etc. Each energy level is still (2l+1) degenerate (due to m – this degeneracy can only be removed by a magnetic field). The energy levels are now described as belonging to shells (labels K, L, M …) which are groups of subshells ( quantum number n, l) which have similar energy. In order of increasing energy. Shell K L Principle n=1 n=2 n=2 n=3 n=3 n=4 n=3 n=4 M N Subshell 1s 2s 2p 3s 3p 4s 3d 4p 6s Energy 5s Degeneracy 1 1 3 1 3 1 5 3 4f 5p 4d 4p 3d 4s N Shell 3p M Shell 3s 2p 2s 1s L Shell K Shell Quantum theory also shows that there is one more property of electrons that has to be considered. Spin The electron behaves as if it were a spinning particle. It has angular momentum (and an associated magnetic moment) independent of its movement through space. In Classical Mechanics, a rotating charged body has a magnetic moment – hence the name Spin. ms=1 2 ms = - 1 2 The electron behaves as if there were 2 possible spin directions – these are dscribed by a quantum number m s spin-up (m s = +1/2) or spin-down (m s = -1/2). An electron in an atom is described by the 4 quantum numbers: n, l, m, m s The Pauli Exclusion Principle states that no two electrons in an atom can have the same set of quantum numbers. Therefore any one orbital can only have 2 electrons in it – with opposite spins. (n, l, m) define the orbital 1s, 2s, 2p x, 2p y, etc. and m s defines the spin. For a ‘many electron atom’ (more than one electron) the ‘ground state’ (lowest energy state) of the atom has the electrons in the orbitals in the following pattern: Z – Atomic Number Symbol Ground State Configuration 1 H 1s1 2 He 1s2 3 Li 1s2 2s1 4 Be 1s2 2s2 5 B 1s2 2s2 2p1 6 C 1s2 2s2 2p2 7 N 1s2 2s2 2p3 8 O 1s2 2s2 2p4 9 F 1s2 2s2 2p5 10 Ne 1s2 2s2 2p6 11 Na 1s2 2s2 2p6 3s1 12 Mg 1s2 2s2 2p6 3s2 13 Al 1s2 2s2 2p6 3s2 3p1 etc .. 3s 2px 2py 2pz 2px 2py 2pz 1s 2s He, Z=2, 1s2 2s 1s C, Z=6, 1s22s22p2 1s Na, Z=11, 1s22s22p63s1 Examples of ground state configurations of atoms. Note: m s indicated by arrow direction. The 2p orbitals do not fill in the order x, y, z – that is just an alphabetic convenience. They do fill spin up first and then down (Hund’s Rule). The orbitals fill in the order: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p … K L M N O P shells This is the order that results in the lowest energy state of the atoms. Example: Mn : Z = 25, 1s2 2s2 2p6 3s2 3p6 4s2 3d5 (count the electrons) The combination of the Pauli Exclusion Principle, the order in which orbitals are filled and Hund’s Rule is called the Aufbau Principle. Building up the electronic configuration of a many electron atom from the the simplest atom – hydrogen. The following is a list of the electronic configurations (electrons in orbitals) of the ground states of the atoms in order of Z.