High-Altitude Medicine by A. Bond
advertisement

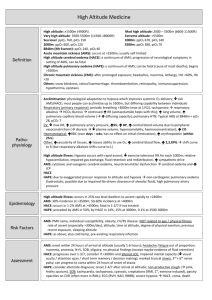
High-Altitude Medicine Alicia Bond MD High altitude Moderate altitude 5,000 – 10,000 feet above sea level Highest U.S. ski resorts High altitude 10,000 – 18,000 feet above sea level High peaks in the lower 48, Europe Extreme altitude Greater that 18,000 feet above sea level Denali, Himalaya, Karakoram, Andes Epidemiology Most cases of high-altitude illness take place in people rapidly ascending to altitudes between 8,000 and 12,000 feet Can affect people who live at low altitude as well as people who live at high altitude and return from travel to lower altitude (re-entry) Millions at risk each year – roughly 20-40% affected by some type of altitude illness 30 million Western states visitors 12,000 Mt. Everest trekkers 1,200 Denali climbers 1 million visitors to extreme high ranges worldwide High-altitude environments Decreased barometric pressure = logarithmically lower partial pressure of oxygen (PO2) in inspired air Higher latitudes have lower barometric pressure at equivalent altitudes Weather systems can significantly lower barometric pressure transiently Cold, dry conditions may be contribute to high-altitude illness Factors affecting risk Rate of ascent Recent high-altitude exposure Genetic variability Sleeping altitude Maximum altitude reached Acclimatization Series of physiologic adaptations to maintain tissue oxygenation Ability to acclimatize varies genetically Hours: Hypoxic ventilatory response (HVR), fluid shift to increase hematocrit, increase in cardiac output Days: Increased erythropoiesis, return of cardiac function to baseline, increase in 2,3-DPG Weeks: Increased plasma volume and red blood cell mass Hypoxic ventilatory response Most important component of acclimatization Affected by genetics, ethanol, sleep medications, caffeine, cocoa, progesterone PaO2 = PiO2 (PaCO2/R) Hyperventilation decreases the partial pressure of CO2 in the alveoli, thereby increasing the partial pressure of oxygen in the alveoli to facilitate oxygenation Resulting metabolic alkalosis slows HVR, and ventilation slowly increases over several days as kidneys excrete bicarb Can be facilitated by acetazolamide People with low HVR at higher risk for illness Cardiovascular Initial increase in resting HR, which normalizes with acclimatization Decrease in maximal heart rate Decrease in plasma volume -> lower stroke volume, increase in hematocrit Shift to extracellular space Diuresis from bicarbonate excretion Decrease in max HR and SV are cardioprotective – myocardial ischemia is rare Hematopoietic response Initial increase in hematocrit due to fluid shift and diuresis Erythropoietin stimulated early, resulting in new RBCs within 4-5 days Over weeks to months, red cell and total circulating volume expand to meet demand Oxygen-hemoglobin curve Above 10,000 feet (PO2 ~ 60), small changes in PO2 cause large changes in SaO2 Initial increase in 2,3diphosphoglycerate (DPG) promotes O2 release to tissues Opposed by respiratory alkalosis, which shifts curve left, favoring oxygen uptake in the lung and higher SaO2 Sleep and periodic breathing Disturbed sleep with less deep sleep and significant arousals common Periodic breathing common Hyperpnea and respiratory alkalosis cause apnea CO2 builds during apnea, causing hyperpnea Not usually associated with significant hypoxemia or high-altitude illness Decreases with acclimatization People with low HVR may have overall regular breathing pattern with periods of more significant apnea and hypoxemia, which are associated with high-altitude illness Acute high-altitude illness Spectrum of disease with intertwining pathophysiology Acute mountain sickness (AMS) High altitude cerebral edema (HACE) High altitude pulmonary edema (HAPE) All correct rapidly with descent Prevention of high-altitude illness Avoid ascent to greater than 8,000 feet in one day Spend 2-3 nights at 8,000-9,000 feet before further ascent Don’t ascend sleeping altitude more than 1500 feet per day Limit exertion, alcohol, and sedative-hypnotics during first days at altitude Day trips to higher altitude while maintaining sleeping altitude can speed acclimatization Acetazolamide 125-250 mg BID Acute mountain sickness Most common with rapid ascent from below 3,000 feet to above 8,000 feet Develops within hours of ascent Headache plus at least one of: Gastrointestinal discomfort Sleep disturbance Generalized weakness or fatigue Dizziness or lightheadedness Headache is usually throbbing, bitemporal, worse at night and with Valsalva AMS: Pathophysiology Pathophysiology incompletely understood Vasodilatory response to hypoxemia, fluid shift, inflammatory mediators, and alterations in cerebrospinal fluid buffering capacity are all implicated No evidence of cerebral edema in AMS, but some studies suggest transient ICP elevations with exertion and Valsalva At risk may be people with low HVR and people with smaller CSF capacity (“tight fit”) Hyperbaria contributes, but role unclear (AMS does not develop with hypoxia alone) AMS: Management Usually resolves within 1-3 days if no additional ascent Mild: Stop ascent, symptomatic treatment, may consider acetazolamide Moderate to severe: Low-flow oxygen, acetazolamide +/- dexamethasone 4 mg q 6 hours, hyperbarics, or descend Immediate descent if s/sx HAPE or HACE Acetazolamide Carbonic anhydrase inhibitor Promotes bicarbonate diuresis and metabolic acidosis, speeding acclimatization Decreases CSF production Maintains oxygenation during sleep Side effects: polyuria and paresthesias 125-250 mg BID for treatment and prevention of AMS High-altitude cerebral edema Least common but most severe form of high-altitude illness Incidence 1-2% of ascents Usually develops above 12,000 feet Usually preceded by AMS and associated with HAPE Most commonly develops days 1-3 after ascent, but can develop later HACE: Presentation Ataxia and altered mentation are hallmarks – ataxia usually first symptom Focal neuro deficits may be present Seizures uncommon but reported Usually preceded by AMS symptoms Any ataxia or change in consciousness in a person at altitude should elicit immediate action! HACE: Pathophysiology Vasogenic cerebral edema caused by same group of mechanisms as AMS (vasodilation, leakage of fluid from vessels) – reversible Increased ICP causes decreased cerebral blood flow, resulting in cell death At advanced stages, cytotoxic edema and necrosis are present - not reversible HACE: Management Immediate descent is key High-flow oxygen and dexamethasone 8 mg (IV, IM, PO) followed by 4 mg q 6 hours if available Hyperbarics may result in temporary improvement but may delay descent Intubation, hyperventilation if severely altered Can try mannitol or furosemide but caution due to dehydration common at altitude HACE: Prognosis If descent initiated early, may be completely reversible over days to weeks without sequelae Reports of ataxia and other neuro deficits persisting months to years Mortality rate greater than 60% if progresses to coma High-altitude pulmonary edema Most common cause of altitude-related death Incidence up to 15% of ascents Usually greater than 10,000 feet, or greater than 8,000 feet with heavy exertion Develops within 2-4 days of ascent, classically on the second night HAPE: Presentation Early signs are severe dyspnea on exertion, fatigue with minimal activity, and dry cough Dyspnea at rest and clear, watery sputum develop as illness progresses Dyspnea at rest is red flag for HAPE and should prompt immediate action! Patchy infiltrates on CXR, worst right middle lobe HAPE: Pathophysiology Hypoxic vasoconstriction causes pulmonary hypertension Uneven vasoconstriction (areas of extreme hypoxia or anatomic difference) causes hyperperfusion of some areas, leading to vascular leak and patchy edema Both hypoxia and pulmonary hypertension are exacerbated by exertion HAPE: Management Symptoms resolve quickly upon descent of 1500-3000 feet Mild cases may be treated with bedrest and O2 to maintain SaO2 > 90 Descent for severe symptoms, minimizing exertion High-flow oxygen Continuous positive airway pressure if available Air drops of O2 may be lifesaving if descent not possible Hyperbarics may help conserve O2 supply Hyperbarics Portable, lightweight, manually-pressurized hyperbaric bags Raise atmospheric pressure 2 psi (103 mmHg) Photo: Rosen’s Emergency Medicine, Courtesy of Thomas Dietz, MD Simulates descent of 4,000-5,000 feet at moderate altitudes, more at higher altitudes Can be lifesaving in HAPE and HACE, relieving symptoms so that patients can descend without evacuation Take-home Slow ascent and acetazolamide are effective in preventing illness Ataxia, altered mentation, and dyspnea at rest are red flags for serious illness Early recognition of HAPE and HACE with descent prevents morbidity and mortality Have fun up there! Key References Marx, JA, ed. Rosen’s Emergency Medicine, 7th Ed. Philadelphia: Mosby Elsevier, 2010 Auerbach, PS, ed. Wilderness Medicine, 6th Ed. Philadelphia: Mosby Elsevier, 2012