Fall 2014
From the Secretary-Treasurer
Sarah McCullough, MD, FACEP
North Dakota
Chapter ACEP
High Altitude Medicine
Lori A. Weichenthal1 and Megann F. Young2
1Associate Professor of
Clinical Emergency Medicine
Director, Wilderness Medicine Fellowship
UCSF-Fresno
2Clinical
Instructor, Department of Emergency Medicine
Fellow, Wilderness Medicine
UCSF-Fresno
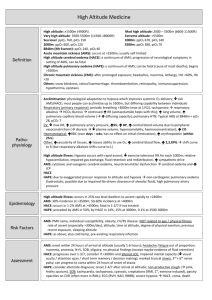
Introduction and Definitions
Many people travel from near sea level to higher elevations every
year in the United States and worldwide for recreation. Travel to
elevations over 2500 meters places people at risk for acute altitude
illness. Primary care physicians may be asked to address issues of
prevention and management of high altitude illness prior to
departure, while physicians working in clinics in these areas or
traveling on expeditions may encounter individuals who are suffering
from these illnesses. This article will address problems related to
ascent to high elevation.
Christopher Boe, MD,
FACEP, President
Contact Us
nd.chapter@acep.org
Phone: 800.798.1822
Ext. 3312
Fax: 972.767.0056
High altitude is defined as elevations between 1500-3500m; very high altitude is defined as
elevations between 3500-5500m; and extreme altitude is defined as elevations greater than
5500m. The higher in elevation a person travels, the greater the risk of altitude illness. Chronic
exposure to high altitude also poses challenges. The world’s highest permanent human habitation
is at 5500m; above this elevation it is not possible to acclimatize to the extreme altitude and
death can occur within days.
High altitude illnesses are those related to the environmental changes of high elevations, primarily
low atmospheric pressure and low oxygen concentration. There is a spectrum of illness, and most
people experience some symptoms over 3000m. There are three major types of altitude illness:
Acute Mountain Sickness (AMS), High Altitude Cerebral Edema (HACE), and High Altitude
Pulmonary Edema (HAPE). Methods of prevention exist and are key, as altitude illness can be
fatal, and the only reasonable treatment for significant disease is descent or evacuation.
Pathophysiology
The physiologic effects of high elevations are primarily related to both low atmospheric pressures
(hypobaria) and low oxygen concentration (hypoxia). Barometric pressure falls as elevation above
sea level increases. As atmospheric pressure falls, the partial pressure of gases in the air
decreases, including oxygen.
As shown in Table 1, there is a corresponding drop in FiO2and SaO2 as elevation increases. This
hypoxemia triggers a number of physiologic changes, detailed in Table 2, including:
Increased sympathetic activity
Increased pulmonary vascular resistance
Increased respiratory rate
Loss of bicarbonate in urine
Increased erythropoietin levels
Increased 2,3 DPG production
The first four physiologic responses listed occur over hours to days. The last two take weeks
before they contribute significantly to a person’s ability to cope with high altitude.
Acclimatization is the process by which people are able to adjust to hypoxia and hypobaria. As
discussed, many physiologic changes occur on ascent to altitude; over time these changes allow
the body to function at high elevations. Arguably the most important factor in acclimatization is
the rate of ascent. A slower ascent allows the body more time to adapt to hypoxia and hypobaria.
It is recommended that the elevation at which one sleeps (“sleeping elevation”) not be increased
by more than 500 meters/day at elevations over 2500 m. A “climb high, sleep low” strategy has
also been suggested, in which people hike or climb higher during the day but descend to a
sleeping elevation not more than 500m higher than the previous day.
Development of illness related to high altitude is basically due to a failure to acclimatize, either
secondary to too rapid an ascent or pre-existing factors in the individual that cause acclimatization
to go awry. Although the exact mechanisms for the development of altitude illness are not fully
understood, what is known is that a complex interaction of the sympathetic response with other
hormones, including aldosterone and arginine vasopressin, cause over-perfusion and leak in the
capillary beds in the brain and lungs resulting in edema (see Figure 1). AMS is a result of mild
edema of the brain and lung while HAPE and HACE develop when the edema becomes severe.
Principal Causes & Risk Factors
Altitude illnesses are unique to those who travel to high elevation, generally over 2500m. The two
major risk factors for developing high altitude illness are rapid ascent and a history of altitude
illness. Epidemiologic studies have suggested a genetic basis for susceptibility to altitude related
illness but such genetic factors have not been well elucidated. Other contributing factors to the
development of altitude illness include: poor conditioning; dehydration; use of drugs or alcohol;
and pre-existing health conditions such as seizure disorder, sickle cell trait or disease, sleep apnea,
pulmonary hypertension, COPD, CHF, and CAD. Prior recent altitude exposure is protective against
altitude illness; even as little as five days of altitude exposure in the previous two months can
decrease acute mountain sickness by as much as 50%.
Keys to History
Keys to history for all forms of altitude illness include: Rate of ascent; degree of acclimatization;
history of prior altitude illness; any preventative medications taken; and comorbid illnesses.
Types of High Altitude Illness (Table 3)
Acute Mountain Sickness (AMS)
1. Keys to history
Like all forms of high altitude illness, the diagnosis of AMS is largely clinical and is based on criteria
adopted at the 1991 International Hypoxia Symposium held at Lake Louise in Alberta, Canada. The
diagnosis of AMS requires recent ascent to altitude (generally >2500m), headache, and at least
one of the following: loss of appetite, nausea, or vomiting; fatigue or weakness; dizziness or
lightheadedness; and/or difficulty sleeping. The headache of acute mountain sickness is classically
bitemporal, throbbing, and worse in the morning and with Valsalva maneuvers or bending over.
2. Physical Examination
There are no specific physical findings to diagnose AMS.
3. Diagnostic Tests
There are no diagnostic tests that help in the diagnosis of AMS.
4. Differential diagnosis and screening
Conditions that can mimic AMS include: dehydration, carbon monoxide poisoning, exhaustion,
hangover, diabetic complications (hypoglycemia, hyperglycemia), hyponatremia, migraine,
infection, hypothermia, viral syndrome.
5. Complications
AMS is an uncomfortable constellation of symptoms that can make a trip to high altitude difficult,
both for the patient and his/her travel companions. It can also cause the patient to make poor
decisions or to have poor physical performance that can lead to injury in this extreme
environment. The worst complication of AMS is that it can progress to HACE, which can be lifethreatening.
6. Natural history and prognosis
The incidence of acute mountain sickness differs by location and elevation. In a 1991 study in
Summit County, Colorado it was found that 22% of people had symptoms of AMS at 1850-2750m,
while 42% had symptoms above 3000m. On Mt. Denali, 50% of people have been noted to have
symptoms, while on Mt. Rainier up to 70% of people have symptoms of AMS. The reason for this
difference is likely related to the rate of ascent: while an ascent of Mt. Denali may take weeks,
many people ascend Mt. Rainier in only two days. Symptoms can occur within two hours of
ascent; they rarely begin more than 36 hours after ascent, and typically will begin to resolve over
the next 24-48 hours at the same elevation. Rapid ascent to higher elevation makes earlier and
more severe symptoms more likely. The majority of patients with AMS who do not continue to
ascend and take time to acclimatize will have resolution of symptoms. For a small group of people
with AMS (< 5 %), their symptoms will progress to HACE which has high morbidity and mortality if
untreated (> 50 %).
7. Treatment
The first step in managing acute mountain sickness is to stop ascent, rest, and allow enough time
to acclimatize to the current elevation. If symptoms resolve with rest, further ascent may be
possible; if symptoms do not resolve, or if they worsen, descent is the only option. If symptoms
resolve with rest and acclimatization and further ascent is planned, it is recommended to start
acetazolamide prior to resuming ascent (see below). Pharmacologic management of AMS may
include the following (see Table 4):
non-opiate analgesics for headache (e.g. ibuprofen, acetaminophen)
antiemetic’s for gastrointestinal symptoms (e.g. ondansetron)
acetazolamide
dexamethasone
Of these medications, only acetazolamide actually speeds acclimatization. All other medications,
including dexamethasone, are only for symptomatic relief.
Prevention is key in the management of all forms of high altitude illness including AMS. Though
some risk factors for altitude illness are non-modifiable, there are several strategies to minimize
the likelihood of developing symptoms. As previously discussed, rate of ascent is among the most
important preventative measures, and an increase in sleeping elevation of no more than
500m/day over 2500m is recommended. Acetazolamide and dexamethasone may also be used for
prophylaxis (Table 4). Patients can be divided into risk categories to predict their likelihood of
developing altitude illness; the approach to prevention should be tailored to each patient’s risk
profile given comorbidities and planned trip (Table 5). For low risk patients, generally no
prophylaxis will be needed. For medium and high risk patients, prophylaxis with acetazolamide
(first-line) or dexamethasone (second-line) should be considered. It is generally not recommended
that acetazolamide and dexamethasone be combined for prophylaxis except in extreme
circumstances (e.g. military or search-and-rescue scenarios where rapid ascent is combined with
immediate strenuous tasks). If travelers are ascending to a high elevation then remaining there
(e.g. flying into Cusco, Peru [3400m/11000ft]), prophylactic medications can be stopped after 2-3
days at elevation. If travelers are ascending to a high elevation then descending, prophylactic
medications should be stopped once descent has begun.
High Altitude Cerebral Edema (HACE)
1. Keys to history
AMS and HACE exist on a spectrum; while some people with HACE will have many of the
symptoms of AMS; the hallmark diagnostic feature of HACE is the presence of neurologic
symptoms. These may be as subtle as mild confusion or change in behavior or as obvious as
lethargy, ataxia, and coma.
2. Physical Examination
The neurologic examination is the key to the diagnosis of HACE. Detection of an alteration in
mental status or ataxia at high altitude is HACE until proven otherwise.
3. Diagnostic studies
In general, high altitude cerebral edema is a clinical diagnosis. Laboratory studies and CSF analysis
are only useful to rule out other diagnoses. If an LP is performed, the opening pressure is likely to
be elevated; case reports exist of opening pressures in excess of 300mmHg. Imaging studies may
also be used to exclude other diagnoses; CT will characteristically show flattened sulci and gyri,
while MRI may demonstrate high T2 signal in the white matter especially on diffusion-weighted
images, but these findings are not pathognomonic for HACE.
4. Differential diagnosis
Conditions that can mimic HACE include: psychosis, carbon monoxide poisoning, diabetic
complication (hypoglycemia, DKA), hyponatremia, stroke/TIA, intoxication, hypothermia, cerebral
mass lesion, and CNS infection. It should be remembered that illness at high altitude is most likely
due to this extreme environment until proven otherwise.
5. Complications
Morbidity from HACE that is untreated is greater than 50 percent. HACE has also been suggested
to contribute too many deaths at high altitude as the confusion and lack of coordination that it
creates lead to bad decisions and errors in highly technical areas of a climb.
6. Natural history
The incidence of HACE is reported to be between 0.1-1 percent overall. As mentioned above,
HACE frequently evolves from AMS. That is why AMS that does not respond to stop in ascent and
rest is so concerning. The progression from AMS to HACE will generally occur over 24-72 hours but
can occur in as little as 12 hours in severe cases. As noted above, untreated HACE has a very high
morbidity and mortality.
7. Treatment
Immediate descent or evacuation to lower elevation is the definitive treatment for HACE. Patients
should descend to an elevation where symptoms resolve, generally at least 1000 meters. Patients
should not descend alone, and should minimize exertion during the descent (carry as little weight
as possible, ride a pack animal if one is available, etc.). If descent is impossible, some treatments
may alleviate symptoms:
Oxygen via nasal cannula to achieve SpO2 at least 90%
A portable hyperbaric chamber (also called a Gamow bag, after its inventor Igor Gamow)
can be used as a temporizing measure. These portable units are made of cloth and have a
foot pump to increase the pressure within the bag to mimic barometric pressure at lower
elevations (Figure 2). Patients who are altered or claustrophobic should not be placed in a
portable hyperbaric chamber; and these should not be used if descent is possible. They are
not easy to use, as the foot pump must constantly be pumped to circulate air and maintain
pressurization.
Acetazolamide (see Table 4)
Dexamethasone (see Table 4)
Prevention strategies and medication prophylaxis are the same for HACE as for AMS.
High Altitude Pulmonary Edema (HAPE)
1. Keys to history
HAPE usually begins 1-3 days after ascent and often occurs at night. Patients may complain of dry
cough, dyspnea at rest, weakness, decreased exercise tolerance, and/or chest tightness or
congestion.
2. Physical examination
The pulmonary examination is key to making this diagnosis. Findings may include crackles,
wheezes, tachypnea, tachycardia, and central cyanosis.
3. Diagnostic studies
4. Like HACE, HAPE is primarily a clinical diagnosis based on known recent ascent to altitude plus
the diagnostic criteria above; laboratory studies and imaging tests may be obtained to rule out
other entities. If obtained, an ECG will typically show sinus tachycardia. Slight leukocytosis is
common. ABG findings include respiratory alkalosis and marked hypoxemia (hypoxemia is
classically out of proportion to physical exam findings; SaO2 values in the 20’s are not
uncommon). CXR may show patchy unilateral or bilateral infiltrates with a normal cardiac
silhouette.
5. Differential diagnosis
6. Conditions that can mimic for HAPE include: asthma, COPD, CHF, bronchitis, pneumonia,
myocardial infarction, and pulmonary embolism.
7. Complications
Left untreated, HAPE can rapidly lead to death due to extreme hypoxia. HAPE is responsible for
the majority of deaths that occur due to altitude illness.
8. Natural history
The incidence of HAPE is estimated to be 0.5-15 % depending on location. Up to 50 % of patients
will have previously had symptoms of AMS. Symptoms occur 1-3 days after ascent, most
commonly at night. Death can occur rapidly without treatment.
9. Treatment
Like HACE, the only definitive treatment for HAPE is descent to an elevation where symptoms
resolve; generally at least 1000 meters. Patients should minimize exertion during the descent
(carry as little weight as possible, ride a pack animal if available, etc.); exertion can worsen
pulmonary edema and hypoxemia. Most patients recover quickly following descent. If descent or
evacuation is not possible, treatments exist to alleviate some symptoms:
oxygen to achieve SpO2 >90%; reducing alveolar hypoxia will immediately decrease
pulmonary hypertension, heart rate, respiratory rate, and improve symptoms
portable hyperbaric chamber (Gamow bag) as above (Figure 2)
nifedipine (Table 4)
Nifedipine acts by decreasing pulmonary vascular resistance, resulting in improved arterial
oxygenation. There is no role for diuretics in the treatment of HAPE, especially because many
patients are already dehydrated. Patients may need to be hospitalized after evacuation if their
symptoms are severe; hospital management includes bed rest and oxygen to maintain O2 sat
>90%. Just as it takes several days to acclimatize to high elevation, it will also take several days to
readjust to sea level; thus appropriate discharge criteria include symptomatic improvement;
radiographic improvement of infiltrates; and room air oxygen saturation >90%; it is not necessary
to wait for the ABG to return to normal as long as patients oxygenate appropriately on room air.
Prevention
As for AMS and HACE, prevention is key. It is recommended that the sleeping elevation not
increase by more than 500m/day at elevations over 2500m (though climbing or hiking higher than
the sleeping elevation during the day may speed the rate of acclimatization). It is also
recommended that overexertion be avoided for the first few days at altitude. Medications may
also be used for prophylaxis (Table 4). In general, preventative drugs are only needed for patients
with a prior history of HAPE. If used, nifedipine should be started the day prior to ascent and
continued until descent begins or the patient has spent five days at same elevation.
Summary Points
Altitude illness is a spectrum of disease related to the body’s inability to acclimatize to an
environment of hypoxia and hypobaria
AMS usually can be managed by rest, stop of ascent and symptomatic treatment
Neurologic signs and symptoms are the hallmark of HACE
Pulmonary signs and symptoms are the hallmark of HAPE
Gradual ascent is the most important factor in preventing altitude illness
Descent is the definitive management of HAPE and HACE
Acetazolamide is the only medication that actually aids in acclimation
Dexamethasone is the primary pharmacologic intervention for HACE
Nifedipine is the primary pharmacologic intervention for HAPE
A portable hyperbaric chamber or oxygen can also help to treat HAPE or HACE if descent is
not possible.
References
Hackett PH, Roach RC. High Altitude Medicine. Wilderness Medicine. Fifth Edition (2007); 2-35.
Luks et al. Wilderness Medical Society Consensus Guidelines for the Prevention and Treatment of
Acute Altitude Illness. Wilderness Environ Med. 2010; 21:146-155.
Hackett PH, Rennie D. The incidence, importance, and prophylaxis of acute mountain sickness.
Lancet. 1976; 2: 1149-1155.
Rupert JL, Koehle MS. Evidence for a genetic basis for altitude related illness. High Alt Med Biol.
2006; 7(2): 150-167.
Luks AM, Swenson ER. Medication and dosage considerations in the prophylaxis and treatment of
high altitude illness. Chest. 2008; 133: 744-755.
Table 1. Altitude, Barometric Pressure, and Oxygenation
Altitude (m)
Atmospheric Pressure (mmHg)
FiO2 (%)
SaO2 (%)
Sea level
760
21%
95%
2800
554
15%
90%
5500
394
10%
76%
8900
252
6%
58%
Table 2. Physiologic changes at high altitude
Altitude Effect
Trigger
Effect of Change
Notes
Increased heart
rate, increased
cardiac output,
fluid retention
Increased
sympathetic
activity
May be adaptive in
conditions such as
pneumonia where hypoxia
Capillary leak, is localized, by decreasing
pulmonary ventilation-perfusion
mismatch. However, in
edema
conditions of global
alveolar hypoxia it is
potentially harmful.
Increased
pulmonary
vascular
resistance
Alveolar hypoxia
induces
pulmonary
vasoconstriction
Increased
respiratory
rate
Increased respiratory rate
can be seen at elevations
as low as 1500m and within
a few hours of arriving at
altitude. Individual
variation exists in the
sensitivity of the carotid
bodies, and studies seem
Carotid bodies
to show that people with
trigger increased Alkalosis due to carotid bodies that are
RR in response to decreased CO2 more sensitive (leading to
hypoxemia
greater increases in RR)
have less altitude illness or
less-severe forms of
altitude illness. Alkalosis
develops due to decreased
carbon dioxide levels; as
alkalosis worsens, the
central respiratory center is
stimulated to slow the
respiratory rate. This
balance may lead to
periodic breathing, in
which people alternate
between rapid breathing
and apnea as the carotid
bodies and central
respiratory center battle
for control
Increased
erythropoietin
levels
Loss of
bicarbonate in
urine
Increased 2,3
DPG
Hypoxemia
Respiratory
alkalosis
Increased erythropoietin
levels are detectable within
Bone marrow two hours of ascent to
produces more altitude; and new red
red blood cells blood cells are found in
circulation 4-5 days after
ascent.
Bicarbonate
diuresis
Takes 24-48 hours for
compensation to occur;
acetazolamide can speed
this process
Shifting the oxyhemoglobin
dissociation curve to the
left makes it easier to
offload oxygen to the
Shifts
tissues. However, alkalosis
oxyhemoglobin (due to hyperventilation)
dissociation counteracts the effects of
curve to the left 2, 3 DPG by shifting the
curve to the right-so at
moderate altitude there is
little net effect on oxygen
dissociation.
Figure 1. Proposed Pathophysiology of High Altitude Illness
Table 3 Types of High Altitude Illness
Type of
Illness
Key Features
Field Treatment
Headache + at least one of: nausea,
vomiting, anorexia, fatigue, weakness,
AMS
dizziness, lightheadedness, difficulty
sleeping
At least 2 of: dyspnea at rest,
cough, weakness, decreased
exercise performance congestion
HAPE PLUS
At least two of: crackles or
wheezes, central cyanosis,
tachypnea, tachycardia
Neurologic symptoms: change in
HACE behavior, confusion, lethargy, ataxia,
coma
Stop ascent, rest
Symptomatic treatment
with analgesics, antiemetics
Acetazolamide 125mg twice
daily
Minimize exertion
Oxygen to maintain O2 sat
>90%
Nifedipine SR 30mg PO
q12h
Hyperbaric therapy or
immediate
descent/evacuation
Minimize exertion
Oxygen to maintain O2 sat
>90%
Acetazolamide 250mg PO
twice daily
Dexamethasone 4mg PO
q12h
Hyperbaric therapy or
immediate
descent/evacuation
Table 4. Drugs used for high altitude illness prophylaxis and treatment
Drug
Class
Mechanism
Indication
of Action
Dosing
Reduces
125mg PO bid
reabsorption
(2.5
AMS, HACE
Carbonic of CO2 in prophylaxis mg/kg/dose for
pediatrics, not
Acetazolamide anhydrae the kidney,
to exceed
inhibitor resulting in
125mg/dose)
bicarbonate AMS, HACE
diuresis and treatment 250mg PO bid
(2.5
metabolic
Notes
First-line
treatment of AMS
and HACE
prophylaxis and
treatment
Start 24 hours
prior to ascent for
best results
Contraindicated in
acidosis. As
a diuretic,
also helps
combat fluid
retention
which
results from
increased
sympathetic
tone
mg/kg/dose for
pediatrics, to
exceed
250mg/dose)
sulfa allergy
Common side
effects: peripheral
paresthesias,
polyuria, nausea,
drowsiness,
impotence,
myopia, altered
flavor of
carbonated
beverages (making
soda and beer
unpalatable)
2 mg PO q6h
or
Dexamethasone Steroid
Unclear
4 mg PO q12h
not used for
prophylaxis in
AMS,HACE
pediatric
prophylaxis
patients
AMS, HACE 8 mg PO once
treatment then 4 mg PO
q12h until
symptoms
resolve
0.15mg/kg/dose
for pediatric
patients
Nifedipine
(sustained
release)
Should not be used
for prophylaxis for
more than 10
consecutive days
Second-line for
AMS and HACE
Use in patients
with
acetazolamide
allergy
Does not improve
acclimatization;
when stopped,
symptoms usually
rebound
Vasodilator;
HAPE
Calcium decreases prophylaxis 30mg PO q12h
channel pulmonary
artery
blocker
HAPE
30mg PO q12h
pressure
treatment
Table 5. Risk assessment for acute mountain sickness for unacclimatized individuals ascending
from <1200m (Adapted from WMS Consensus Guidelines).
Category
Factors
Low Risk
Moderate
Risk
High Risk
No prior h/o altitude illness,
ascending to less than 2800m
Ascending to 2500-3000m over
2 or more days with subsequent
increase in sleeping elevation
<500m/d
Prior h/o AMS ascending to
>2500m in 1 day
No h/o AMS ascending to
>2800m in 1 day
Increasing sleeping elevation
>500m/day above 3000m
h/o AMS ascending to >2800 in
1 day
All individuals with prior h/o
HACE or HAPE
Ascending to >3500m in 1 day
Increasing sleeping elevation
>500/day above 3500m
Very rapid ascents (e.g.
Mt.Kilimanjaro)
Figure 2. Portable hyperbaric chamber
Recommendations
Gradual ascent
No medications necessary
Consider prophylaxis with
acetazolamide (first line) or
dexamethasone (second line)
Strongly consider prophylaxis
with acetazolamide (first
line) or dexamethasone
(second line)
ACEP14: Emergency Medicine's Conference
With more than 350 educational sessions, interactive workshops, skills labs, the world’s largest
Exhibit Hall and numerous social networking opportunities, ACEP14 has something for every
emergency physician. Then, there’s Chicago. Consider it the icing on your conference cake.
The nation’s third largest city offers some of the finest restaurants in the world, and a handful of
those are offered this year in the ACEP14 Dine Around Program. Chicago is also considered the
cultural capital of the Midwest, and two of those attractions are mixed in with the opening and
closing ceremonies during this year’s conference.
Emergency Consultants, Inc., presents the ACEP14 Kickoff Party from 7:30 p.m.-Midnight on
Monday, Oct. 27, at the Navy Pier Grand Ballroom. The Closing Celebration, presented by EMCare,
is from 7-11 p.m. on Wednesday, Oct. 29, at the spectacular Museum of Science and Industry! MSI
is the largest science center in the Western Hemisphere with more than 35,000 artifacts and
nearly 14 acres of hands-on experiences.
Wrapped between these events is emergency medicine education that grows each year.
Critical care, pediatrics, health policy, pulmonary disorders – these are only a few of the 26 areas
of practice you can elevate at this event and improve patient care. Get it started at 8 a.m.
Monday, Oct. 27, at the Opening Session, where keynote speakers Steven Levitt and Stephen
Dubner, authors of international best-sellers Freakonomics and SuperFreakonomics, will look at
the economics of health care. Levitt is the William B. Ogden Distinguished Service Professor of
Economics at the University of Chicago. Dubner is an award-winning author, journalist, and TV and
radio personality. The annual Colin C. Rorrie, Jr. Lecture is on the same day at 12:30 p.m., and will
be delivered by AMA President-Elect Steven Stack, MD, FACEP. Dr. Stack with talk about “The
ACA: The Rocky Road to Health Reform.”
Part of education is learning about new products and technology.
There’s plenty of that in the Exhibit Hall. With more than 500 exhibitors and the sophomore year
of innovatED, you’ll see the latest technologies, solutions, products and services from some of the
most respected names in the emergency medicine. Back by popular demand is the new ACEP
Resource Center. This one-stop shop showcases information on a variety of ACEP tools, benefits
and services, as well as emergency medicine issues. ACEP leaders and staff members will be
available to answer your questions, discuss college policy and direction, and provide information
on products and resources from ACEP. The Exhibit Hall and innovatED are opens at 9:30 a.m. on
Monday, Tuesday and Wednesday.
Several options are available even before ACEP14 officially kicks off.
The pre-conference schedule is packed, highlighted by the Procedural Cadaver-Based Skills Labs
and the Advanced Invasive Procedural Skills Lab. Also new to the list of events held in conjunction
with ACEP14 is the new EM Hackathon on Oct. 24-26. ACEP and EMRA have teamed up with
Hacking Medicine at MIT and Chicago Health 2.0 to offer an emergency medicine problem-solving
challenge during ACEP14. The EM Hackathon leverages out-of-the-box thinkers for an amped-up,
all-weekend, problem solving session where emergency medicine physicians collaborate with
computer programmers, engineers, and other subject matter experts to tackle challenges in EM.
The Hackathon will begin on Friday evening, October 24 at 1871 (map), just before
the ACEP14 annual meeting in Chicago. Participants will work (hack) through the weekend and
judges will decide the winners on Sunday, October 26.
Whether you can get there early enough for pre-conference events and Hackathon fun, or show
up just in time for the Opening Session, ACEP can guarantee a packed schedule of possibilities and
top-notch education. Make the most of it, and we’ll see you in Chicago.
Clinical News
DEA Moves Hydrocodone Combination Products to Schedule II
The Drug Enforcement Administration is making it harder to prescribe hydrocodone combination
products.
The move was expected, as the agency proposed in February to move hydrocodone combinations
from schedule III to schedule II in response to requests from both the U.S. Department of Health
& Human Services and the Food and Drug Administration.
Read the entire article
Five Factors Predict Biphasic Reactions in Children With Anaphylaxis
“Children who match none of the five criteria can actually be discharged sooner from the ED
[emergency department]. These predictors can potentially improve the efficiency and quality of
care in the ED,” Dr. Waleed Alqurashi said at the annual meeting of the Society for Academic
Emergency Medicine.
Read the entire article
Attention to Risk Factors Could Reduce CAP-Related Emergency Revisits
Fever or lack of an antibiotic prescription are two factors that increase the risk of a return visit to
the emergency department and subsequent hospital admission in children with communityacquired pneumonia, according to a review of ED medical records.
Read the entire article
Welcome New Members
Kevin J. Torkelson
Jacqueline Huber
North Dakota Chapter ACEP, PO BOX 619911, Dallas, TX 75261-9911
Copyright © 2014 North Dakota Chapter ACEP. All rights reserved.
Unsubscribe