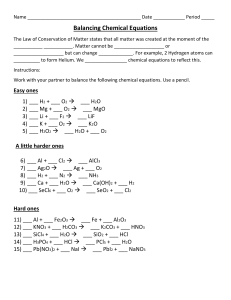
Types of Reactions Name:_______________________ Period: _____ Identify each as either: synthesis, decomposition, single replacement or double replacement reactions. 1. ____________________________ N2 + 3 H2 2 NH3 2. ____________________________ 2 Li + 2 H2O 2 LiO + H4 3. ____________________________ 2 NaNO3 2 NaNO2 + O2 4. ____________________________ 2 C6H14 + 19 O2 12 CO2 + 14 H2O 5. ____________________________ H2CO3 CO2 + H2O 6. ____________________________ SO2 + H2O H2SO3 7. ____________________________ 3 Fe + 4 H2O Fe3O4 + 4 H2 8. ____________________________ 2 KI + Pb(NO3)2 PbI2 + 2 KNO3 9. ____________________________ CaO + SO2 CaSO3 10. ____________________________ 2 HgO 2 Hg + O2 Use the Activity Series to determine if the following reactions will occur. Simply write a “yes” or a “no” in the space next to the reactants. *BONUS* Predict the products (of the reactions that will occur) and balance the equation for extra points! 11. Na + MgBr2 ____________________________________________________________________________ 12. Ni + MnS _____________________________________________________________________________ 13. Cu + Pb(NO3)2 __________________________________________________________________________ 14. Zn + SnCl2 ______________________________________________________________________________ 15. Ca + K2O ________________________________________________________________________________