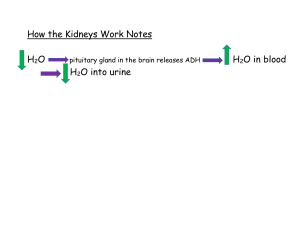
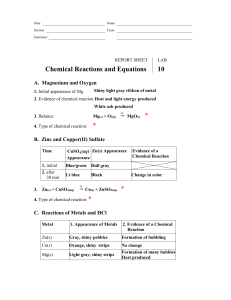
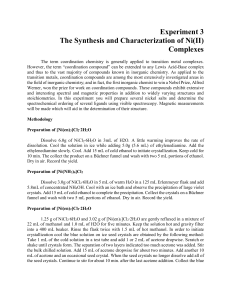
Name ___________________________________________ Date ____________ Period _____ Balancing Chemical Equations The Law of Conservation of Matter states that all matter was created at the moment of the ___________ ___________. Matter cannot be ___________________ or ___________________ but can change _____________. For example, 2 Hydrogen atoms can __________ to form Helium. We ________________ chemical equations to reflect this. Instructions: Work with your partner to balance the following chemical equations. Use a pencil. Easy ones 1) 2) 3) 4) 5) ___ H2 + ___ O2 ___ H2O ___ Mg + ___ O2 ___ MgO ___ Li + ___ F2 ___ LiF ___ K + ___ O2 ___ K2O ___ H2O2 ___ H2O + ___ O2 A little harder ones 6) ___ Al + ___ Cl2 ___ AlCl3 7) ___ Ag2O ___ Ag + ___ O2 8) ___ H2 + ___ N2 ___ NH3 9) ___ Ca + ___ H2O ___ Ca(OH)2 + ___ H2 10) ___ SeCl6 + ___ O2 ___ SeO2 + ___ Cl2 Hard ones 11) ___ Al + ___ Fe2O3 ___ Fe + ___ Al2O3 12) ___ KNO3 + ___ H2CO3 ___ K2CO3 + ___ HNO3 13) ___ SiCl4 + ___ H2O ___ SiO2 + ___ HCl 14) ___ H3PO4 + ___ HCl ___ PCl5 + ___ H2O 15) ___ Pb(NO3)2 + ___ NaI ___ PbI2 + ___ NaNO3