Net Ionic Reactions Practice Problems with Answers
advertisement

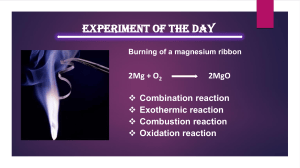
Net Ionic Reactions – Practice #5 Do NOT submit the directions and questions to the dropbox, only the answers! For each of the following reactions, write a balanced equation for the reaction in part (i) and answer the question about the reaction in part (ii). In part (i), coefficients should be in terms of lowest whole numbers. Assume that solutions are aqueous unless otherwise indicated. Represent substances in solutions as ions if the substances are extensively ionized. Omit formulas for any ions or molecules that are unchanged by the reaction. FYI, here is how each question is scored: 1 point is earned for the correct reactants. 2 points are earned for the correct products. 1 point is earned for correctly balancing the equation for both mass and charge. 1 point is earned for the correct answer or observation for part ii. Questions: 1. (i) A small piece of sodium is placed in a beaker of distilled water. (ii) The reaction is exothermic, and sometimes small flames are observed as the sodium reacts with the water. Identify the product of the reaction that burns to produce the flames. 2Na + H2O H2 + 2Na+ + 2 OHH2 2. (i) Solid ammonium carbonate decomposes as it is heated. (ii) Predict the algebraic sign of ∆So for the reaction. Explain your reasoning. (NH4)2CO3 2NH3 + CO2 + H2O + ∆So because the disorder increases (4 molecules are made from 1) 3. (i) Sulfur trioxide gas is bubbled into a solution of sodium hydroxide. (ii) Is the temperature of the mixture likely to increase or decrease? SO3 + 2 OH- SO42- + H2O --the temperature should increase as for any neutralization reaction 4. (i) A solution containing silver (I) ion (an oxidizing agent) is mixed with a solution containing iron (II) ion (a reducing agent). (ii) If the contents of the reaction mixture described above are filtered, what substance(s), if any, would remain on the filter paper? Ag+ + Fe2+ --> Ag + Fe3+ The precipitated solid silver will remain on the filter paper. 5. (i) A hydrolysis reaction occurs when solid sodium sulfide is added to distilled water. (ii) Indicate whether the pH of the resulting solution is less than 7, equal to 7, or greater than 7. Explain. Na2S + H2O → 2 Na+ + HS- + OH- OR Na2S + 2 H2O → 2 Na+ + H2S + 2 OHThe pH of the resulting solution is greater than 7. The hydrolysis reaction of S2produces the base OH-, thus raising the pH above 7. 6. (i) Solutions of sodium phosphate and calcium chloride are mixed. (ii) What is the most likely color of the final reaction mixture? Explain. 3 Ca2+ + 2 PO43- Ca3(PO4)2 --white; the absence of transition metals makes this likely 7. (i) Hydrobromic acid is added to a solution of potassium hydrogen carbonate. (ii) When a gas produced by the reaction is bubbled through limewater, what visible change is expected? HCO3- + H+ CO2 + H2O --limewater becomes cloudy when carbon dioxide contacts it due to the formation of CaCO3 8. (i) A solution of sulfuric acid is added to a solution of barium hydroxide. (ii) If equimolar amounts of the two reactants are mixed, what change will be indicated by a conductivity device? 2H+ + SO42- + Ba2+ + 2OH- BaSO4 + 2H2O --if equal moles are mixed “all” of the ions will be combined in either a precipitate or water and thus the conductivity should be essentially zero 9. (i) Solid cesium oxide is added to water. (ii) Describe the effect of the resulting mixture on litmus paper. Cs2O + H2O 2 Cs+ + 2 OH--the litmus paper will turn blue because of the presence of OH10. (i) Zinc metal is added to a hydrobromic acid solution. (ii) Write the oxidation half-reaction for the reaction. Zn + 2HBr ZnBr2 + H2 Zn + 2H+ + 2Br- Zn+2 + 2 Br- + H2 Zn + 2 H+ Zn+2 + H2 Zn Zn+2 + 2 e-